Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
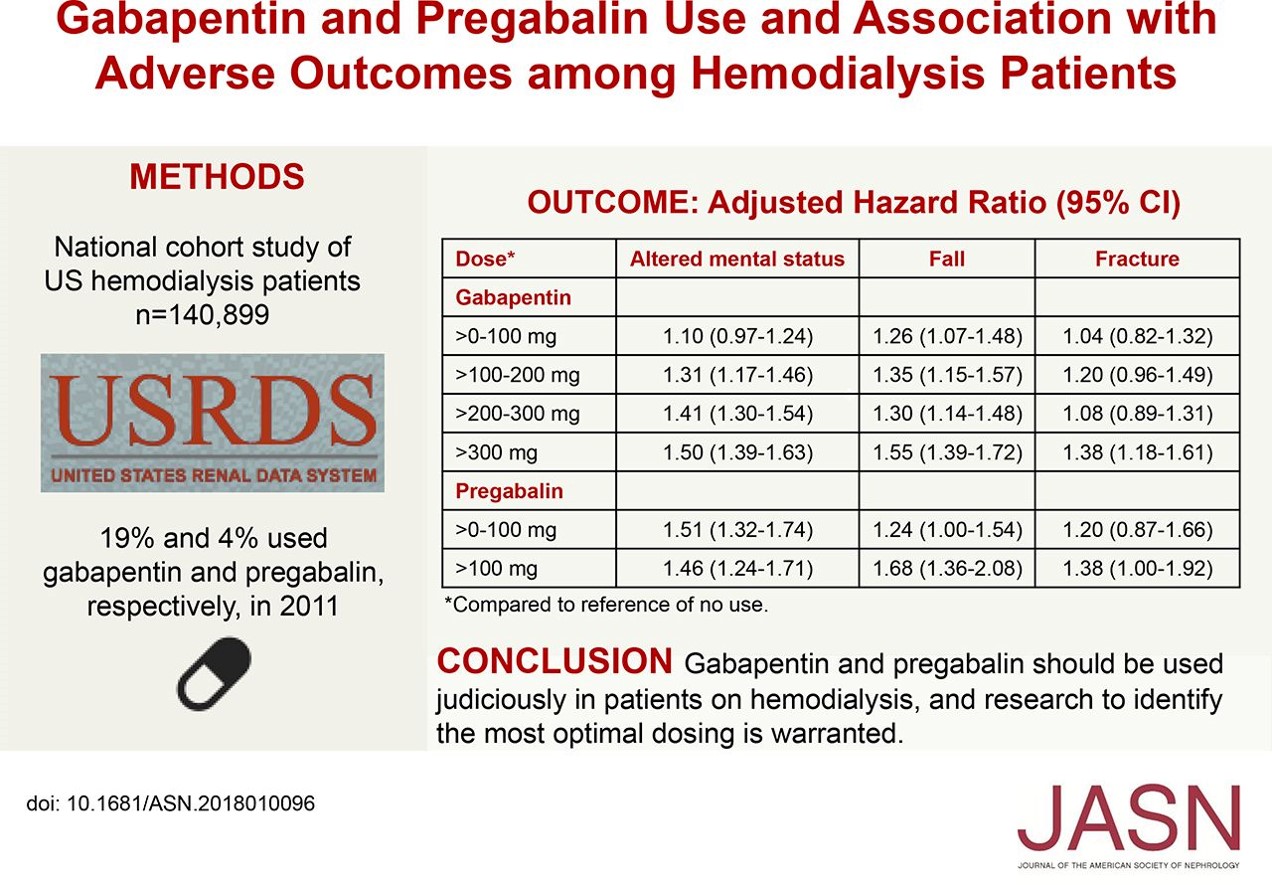
approximately 3 days. The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. TABLE 1. Gabapentin Oral Solution Dosage Based on Renal Function. regarding Gabapentin Oral Solution, please call 1-800-541-4802. What are the ingredients in Gabapentin Oral Solution? Active ingredient: gabapentin 250 mg/5 mL The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin Oral Solution, 473 ml (Xylitol Free) , RX GENERICS, Manufacturer Item #:65162069890, Patterson Item #:07-893-5342 The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic acid with a molecular formula of . C. 9 H 17 NO 2 and a molecular weight of 171.24. The structural formula of gabapentin is: Gabapentin is a human prescription drug by Amneal Pharmaceuticals Llc. The product is distributed in 4 packages with NDC codes 65162-102-03, 65162-102-10, What are the ingredients in Gabapentin Oral Solution? Active ingredient: gabapentin 250 mg/5 mL The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Complete details for NDC 65162-0698-90 Gabapentin 250 mg/5mL including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030002020. What are the ingredients in Gabapentin Oral Solution? Active ingredient: gabapentin 250 mg/5 mL. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. This Medication Guide has been approved by the U.S. Food and Drug Administration. What are the ingredients in gabapentin oral solution? Active ingredient: gabapentin, USP . Inactive ingredients in the oral solution: acesulfame potassium, carboxymethylcellulose sodium, magnasweet, peppermint flavor, potassium sorbate and strawberry anise artificial flavor. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH. Gabapentin capsules, for oral use Gabapentin tablets, for oral use Gabapentin oral solution Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin is indicated for: • • DOSAGE AND ADMINISTRATION • • • DOSAGE FORMS AND STRENGTHS • • • CONTRAINDICATIONS Known hypersensitivity to gabapentin or its ingredients (4) WARNINGS AND We are also excited about our work to bring patients more affordable biologic therapy options through our Biosciences, which includes a broad portfolio of institutional injectables as well as Amneal’s growing biosimilars portfolio. And we’re expanding our business across select international markets. Learn more about our product portfolios: Material Name: Gabapentin Oral Solution, 250 mg / 5 mL _____ Page 2 of 5 Issue Date: 02/16/12 Revision: 1.0000 Print Date: 2/16/2012 . First Aid: Inhalation. Remove the person from the exposed area to fresh air immediately. Get medical advice if adverse symptoms appear. Gabapentin is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Amneal Pharmaceuticals Llc. The primary component is Gabapentin. Sample Package? 65162-698 National Drug Code registration, ingredients, and packaging details. The NDC code 65162-698 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. What are the ingredients in Gabapentin Oral Solution? Active ingredient: gabapentin 250 mg/5 mL. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. This Medication Guide has been approved by the U.S. Food and Drug Administration. 2.1 Dosage for Postherpetic Neuralgia . In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). pharmacist for information about gabapentin oral solution that was written for healthcare professionals. For more information go to www.amneal.com. or call 1-877-835-5472. What are the ingredients in gabapentin oral solution? Active ingredient: gabapentin, USP What are the ingredients in gabapentin oral solution? Active ingredient: gabapentin. Inactive ingredients in the oral solution: carboxymethylcellulose sodium, methylparaben, propylene glycol, propylparaben, purified water, xylitol, anise flavor, artificial strawberry flavor and hydrochloric acid added for adjustment of pH.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |