Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
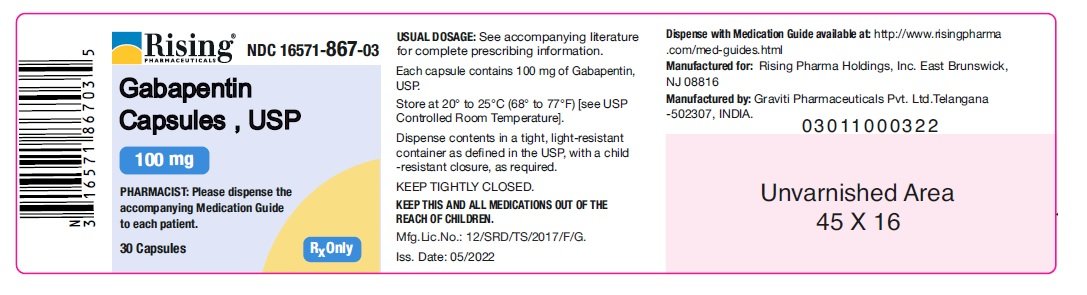
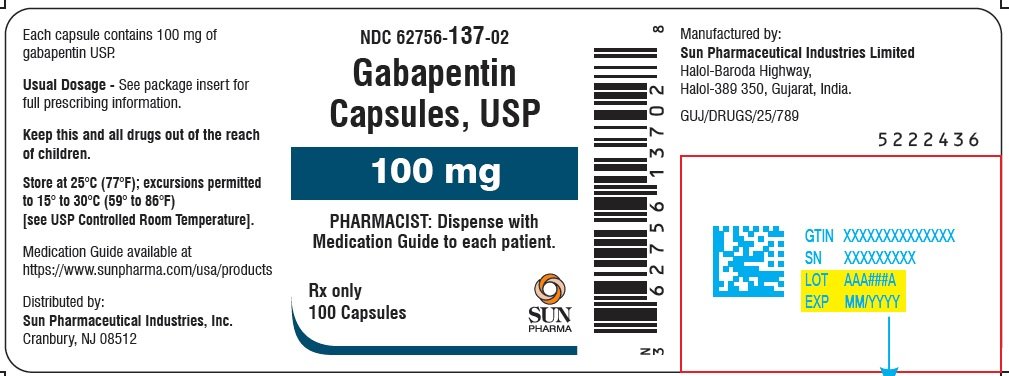
What is NDC 65162-698? The NDC code 65162-698 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Order Gabapentin 250 mg / 5 mL Solution 473 mL (16 oz.) by Amneal 65162069890. Call Us. Customer Service 855.571.2100. Need help with SupplyManager? 800.422.0280. Accounts Receivable 2.1 Dosage for Postherpetic Neuralgia. In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Moreover, because gabapentin causes somnolence and dizziness [see Warnings and Precautions (5.4)], patients should be advised not to operate complex machinery until they have gained sufficient experience on gabapentin to assess whether gabapentin impairs their ability to perform such tasks. 5.4 Somnolence/Sedation and Dizziness Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy (1) Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Amneal Pharmaceuticals of New York LLC: Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the zTake Gabapentin Oral Solution exactly as prescribed. Your healthcare provider will tell you how much Gabapentin Oral Solution to take. {Do not change your dose of Gabapentin Oral Solution without talking to your healthcare provider. zGabapentin Oral Solution can be taken with or without food. If you take an antacid containing Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. TABLE 1. Gabapentin Oral Solution Dosage Based on Renal Function. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. We are also excited about our work to bring patients more affordable biologic therapy options through our Biosciences, which includes a broad portfolio of institutional injectables as well as Amneal’s growing biosimilars portfolio. And we’re expanding our business across select international markets. Learn more about our product portfolios: Patients taking Gabapentin Oral Solution should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Gabapentin, USP is a white to off-white crystalline powder. It is freely soluble in water and in alkaline and acidic solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is −1.25. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy GABAPENTIN- gabapentin tablet, film coated GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |