Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
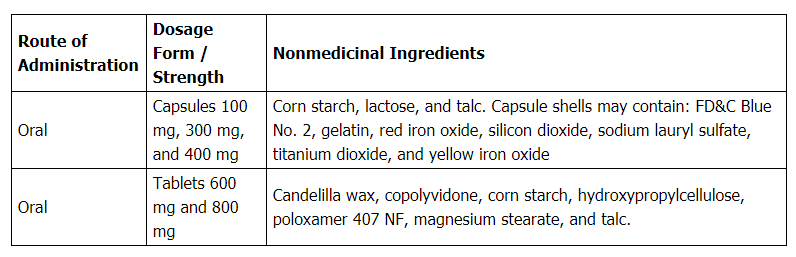
Gabapentin Capsules 400 mg – 100/Bottle. Overview. Reference Brand: February 27, 2025 | Aurobindo Receives FDA Approval for Tretinoin Gel USP, 0.025% Gabapentin Tablets USP 600 mg – 100/Bottle. Overview. Reference Brand: 2025 | Aurobindo Receives FDA Approval for Nystatin Cream USP, 100,000 units per gram It is supplied by Aurobindo Pharma. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 100 mg is not a controlled substance under the Controlled Substances Act (CSA). Stopping gabapentin capsules suddenly can cause serious problems. Gabapentin capsules can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin capsules may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Gabapentin bioavailability (fraction of dose absorbed) tends to decrease with increasing dose. Absolute bioavailability of a 300 mg capsule is approximately 60%. Food, including a high-fat diet, has no clinically significant effect on gabapentin pharmacokinetics. Gabapentin pharmacokinetics are not affected by repeated administration. Gabapentin Aurobindo 100 mg/ 300 mg/ 400 mg καψάκια, σκληρά: Hongarije: Gabapentin Aurobindo 100 mg/ 300 mg/ 400 mg kemény kapszula: Ierland: Gabapentin Aurobindo 100 mg/ 300 mg/ 400 mg capsules, hard: Italië: Gabapentin Aurobindo 100 mg/ 300 mg/ 400 mg capsule rigide: Letland: Gabapentin Aurobindo 100 mg/ 300 mg/ 400 mg cietās Pill with imprint D 03 is Yellow, Capsule/Oblong and has been identified as Gabapentin 300 mg. It is supplied by Aurobindo Pharma. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin by is a Prescription medication manufactured, distributed, or labeled by New Horizon Rx Group, LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow. The active ingredient in gabapentin capsules is gabapentin, which has the chemical name 1-(aminomethyl) cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. Gabapentin Capsules 300 mg – 100/Bottle. Overview. Reference Brand: February 27, 2025 | Aurobindo Receives FDA Approval for Tretinoin Gel USP, 0.025% TABLE 1. Gabapentin Capsules Dosage Based on Renal Function TID = Three times a day; BID = Two times a day; QD = Single daily dose a For patients with creatinine clearance <15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min These highlights do not include all the information needed to use GABAPENTIN TABLETS safely and effectively. See full prescribing information for GABAPENTIN TABLETS. GABAPENTIN tablets, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin tablets are indicated for: Postherpetic neuralgia in adults (1) Aurobindo Pharma Limited: Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial Aurobindo Pharma Limited: Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial Stopping gabapentin capsules suddenly can cause serious problems. Gabapentin capsules can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin capsules may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Pill with imprint D 02 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by Aurobindo Pharma. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. It is supplied by Aurobindo Pharma. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 300 mg is not a controlled substance under the Controlled Substances Act (CSA). Gabapentin Capsules 100 mg – 1000/Bottle. Overview. Reference Brand: 2025 | Aurobindo Receives FDA Approval for Nystatin Cream USP, 100,000 units per gram These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin capsules are indicated for: Postherpetic neuralgia in adults (1) are allergic to gabapentin or any of the other ingredients in gabapentin tablets. See the end of this Medication Guide for a complete list of ingredients in gabapentin tablets. Before taking gabapentin tablets, tell your healthcare provider about all of your medical conditions including if you: have or have had kidney problems or are on
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |