Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
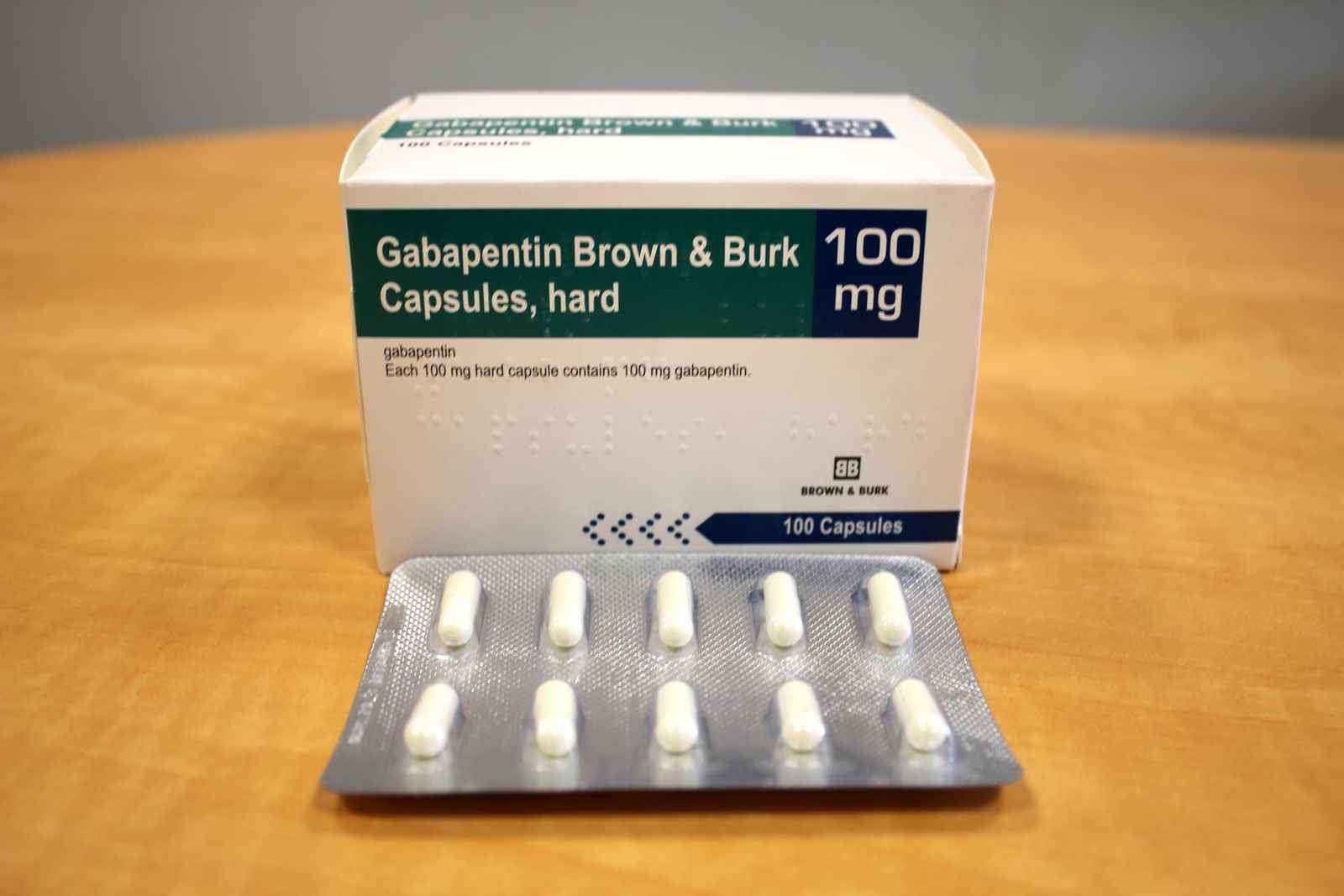
brand name molecule dosage form; aci free (lemon) svarjiksara 2.904g + nimbukamlam 2.015g + sodium saccharin 12 mg/5g: powder: aci free (pudina) svarjiksara 2.913g + nimbukamlam 2.041g + sodium saccharin 15 mg/5g The NDC code 69097-814 is assigned by the FDA to the product Gabapentin which is product labeled by Cipla Usa Inc.. The product's dosage form is . The product is distributed in 2 packages with assigned NDC codes 69097-814-07 100 capsule in 1 bottle , 69097-814-12 500 capsule in 1 bottle . gabapentin tablets is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer gabapentin tablets three times a day using 600 mg or For more information about gabapentin capsules, or to report side effects regarding gabapentin capsules, please call Cipla Ltd. at 1-866-604-3268. What are the ingredients in gabapentin capsules? Active ingredient: Gabapentin, USP. Inactive ingredients: The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral capsule. Gabapentin capsules are indicated for: • Management of postherpetic neuralgia in adults • Coadministration of gabapentin with hydrocodone decreases hydrocodone exposure Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy GABAPENTIN is a Oral Capsule in the Human Prescription Drug category. It is labeled and distributed by Cipla Usa Inc.. The primary component is Gabapentin. Sample Package? 69097-943 National Drug Code registration, ingredients, and packaging details. The User acknowledges that Cipla: (a) has no control of or responsibility for the User’s use of the Medication Guides or content provided herein, (b) has no knowledge of the specific or unique circumstances under which the Medication Guides or content provided thereon may be used by the User, and (c) has no liability to any person for any The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin,which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. Gabapentin is a human prescription drug by Cipla Usa Inc.. The product is distributed in 2 packages with NDC codes 69097-815-07, 69097-815-12.Gabapentin is use What are the ingredients in gabapentin capsules, USP? Active ingredient: gabapentin, USP. Inactive ingredients in the capsules: corn starch, gelatin, magnesium stearate, mannitol, sodium lauryl sulphate, talc, titanium dioxide, black edible ink which contains iron oxide black, potassium hydroxide, propylene glycol, and shellac. The NDC Packaged Code 69097-814-12 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, labeled by Cipla Usa Inc.. The product's dosage form is and is administered via form. Is NDC 69097-814 included in the NDC Directory? Cipla Each capsu e contains 100 mg of gabapentin, USP DOSAGE AND USE: See package insert for fu prescribing information store at to (680 to 770F) [See USP Controlled Room Temperature]. Manufactured by: nvaGen Pharmaceutica s, nc. (a subsidiary Of Cipla Ltd.) Hauppauge, NY 11788 Manufactured for: Cipla USA, Inc. 10 Independence Boulevard, Suite 300 Gabapentin Tablets USP, 800 mg are white, elliptical, film-coated scored tablets debossed “O|E” on one side and “800” on the other side; 4 CONTRAINDICATIONS. Gabapentin is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS gabapentin Capsules is 300 mg to 600 mg three times a day. Dosages up to 2,400 mg/day have been well tolerated in long-term clinical studies. Doses of 3,600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer gabapentin capsules three times a day using effects regarding gabapentin capsules, please call Cipla Ltd. at 1-866-604-3268. What are the ingredients in gabapentin capsules? Active ingredient: Gabapentin, USP Inactive ingredients: The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100mg capsule shell contains titanium dioxide. The 300mg capsule contains FD&C Red Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). Gabapentin is in a class of medications called anticonvulsants. What are the ingredients in gabapentin? Active ingredient: Gabapentin USP . Inactive ingredients in the capsules: anhydrous lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin, sodium lauryl sulfate, and titanium dioxide. Gabapentin Capsules, USP are supplied as imprinted hard gelatin capsules containing 100 mg, 300 mg and 400 mg of gabapentin, USP. The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide. The 300 mg capsule contains FD&C Red 40, D&C Yellow 10 and titanium dioxide. Gabapentin is a human prescription drug by Cipla Usa Inc.. The product is distributed in 2 packages with NDC codes 69097-813-07, 69097-813-12.Gabapentin is use
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |