Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
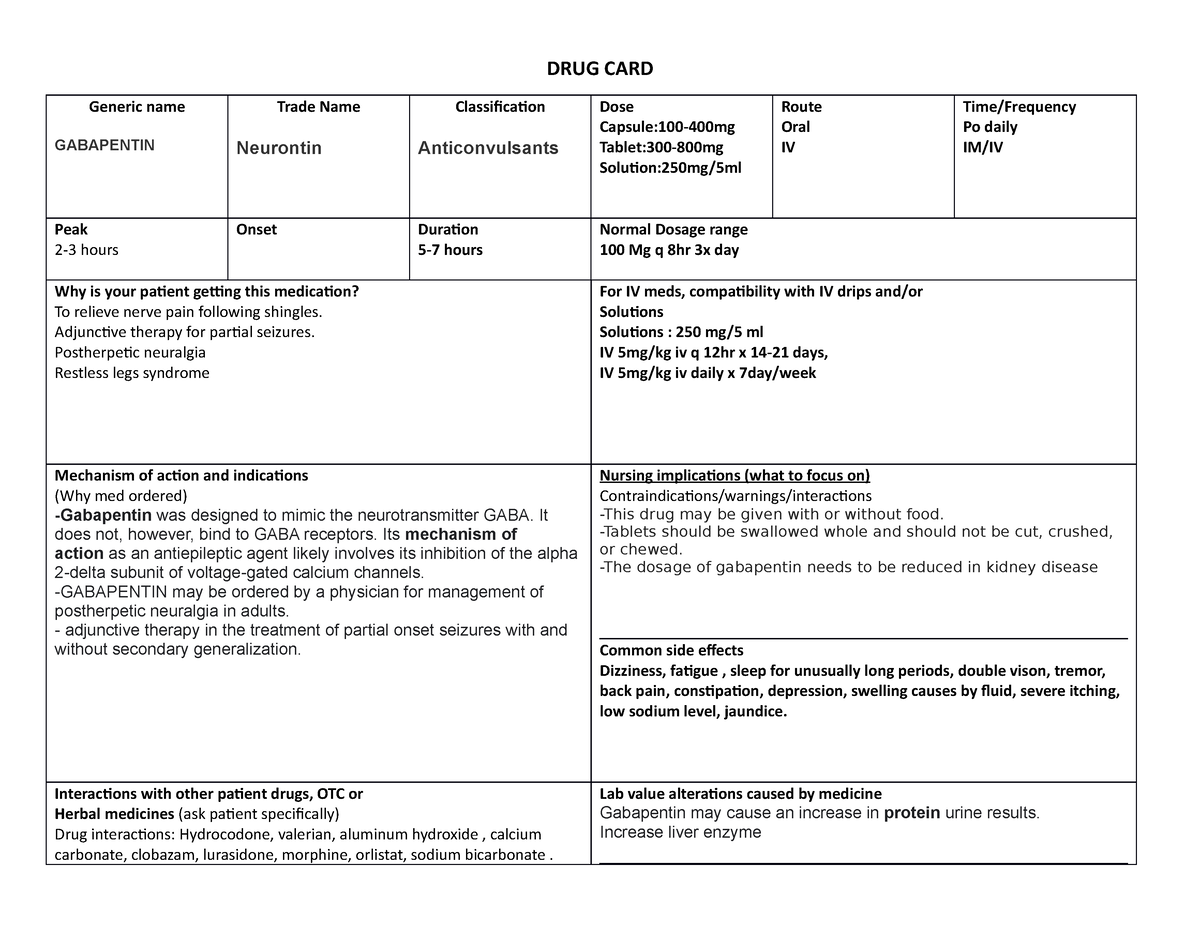 |  |
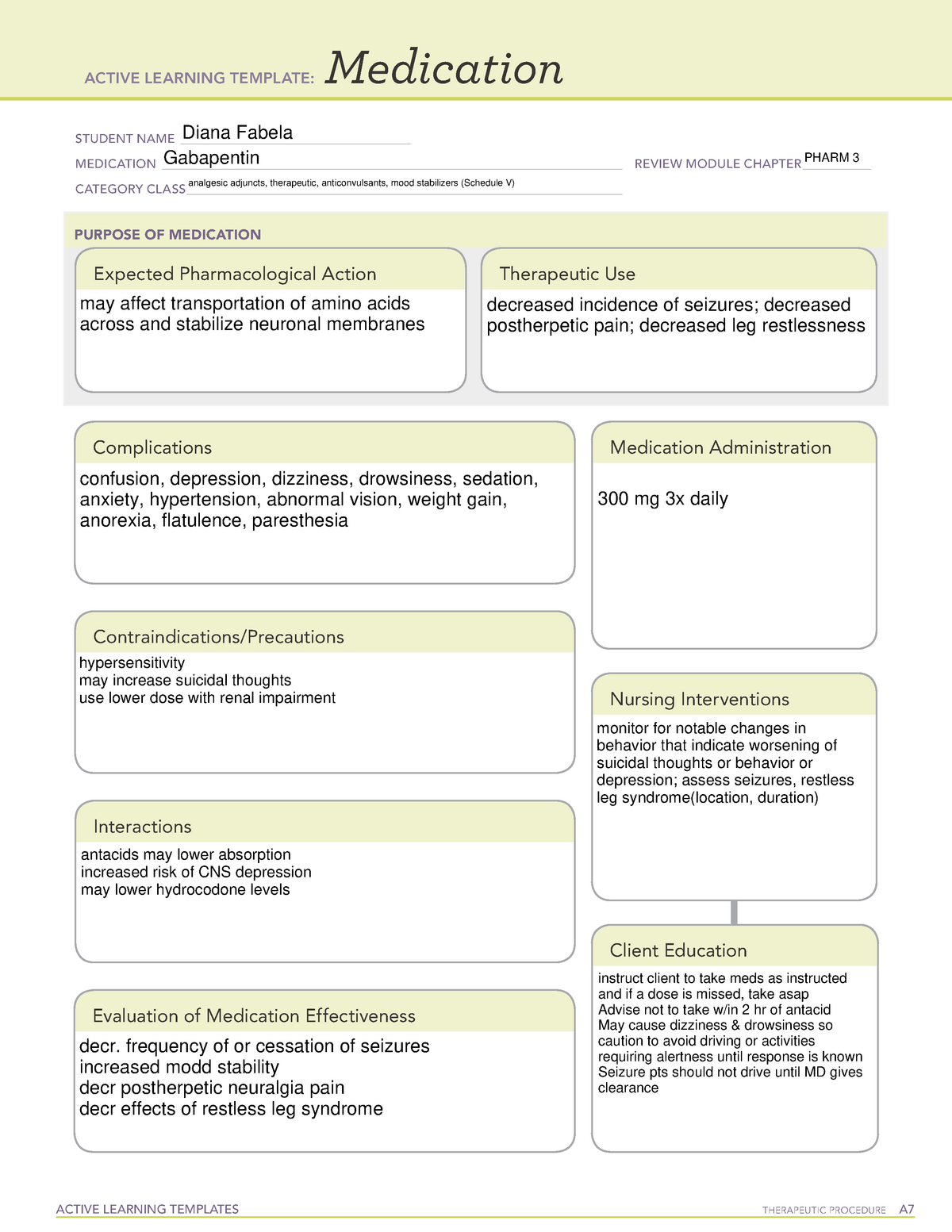
Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Neurontin is used in adults to treat neuropathic pain (nerve pain) caused by herpes virus or shingles (herpes zoster). Neurontin is also used to treat seizures in adults and children who are at least 3 years old. Use only the brand and form of gabapentin your doctor has prescribed. (a) Any compound, mixture, or preparation containing any of the following limited quantities of narcotic drugs, which shall include one or more nonnarcotic active medicinal ingredients in sufficient proportion to confer upon the compound, mixture, or preparation valuable medicinal qualities other than those possessed by the narcotic drug alone: Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Gabapentin belongs to a class of drugs called anticonvulsants. These drugs are used to treat a variety of conditions caused by abnormal electrical activity in the brain, including seizures, nerve pain, and restless leg syndrome. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Gabapentin is in a class of medications called anticonvulsants. Gabapentin treats seizures by decreasing abnormal excitement in the brain. Gabapentin relieves the pain of PHN by changing the way the body senses pain. Gabapentin is a prescription drug most commonly prescribed to relieve nerve pain following shingles in adults, treating the pain of post herpetic neuralgia. Gabapentin belongs to a class of drugs known as anti- seizure drugs. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada, or Australia, and the other gabapentinoids, including phenibut, are not controlled substances Drug Interactions. Gabapentin can interact with various medications, natural products, and food. It is important to be aware of these potential drug interactions. Drug-Drug. Gabapentin can potentially interact with several other medications, and it is important to be aware of these drug-drug interactions. Here are some examples: 1. Opioids. Patients exposed to gabapentin during pregnancy are encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334. Additional information is available at www.aedpregnancyregistry.org . Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Information System (NFLIS) Drug database collects scientifically verified data on drug items and cases submitted to and analyzed by participating federal, state and local forensic , drug laboratories. NFLIS-Drug received 3,614 reports of gabapentin in 2019; 3,348 in 2020; 3,128 What class of drug is gabapentin? Gabapentin has been a federally noncontrolled substance since its FDA approval in 1993. It’s typically used for epilepsy and nerve pain, a severe symptom that Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Find information on Gabapentin (Gralise, Horizant) in Davis’s Drug Guide including dosage, side effects, interactions, nursing implications, mechanism of action, half life, administration, and more. Davis Drug Guide PDF.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |