Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
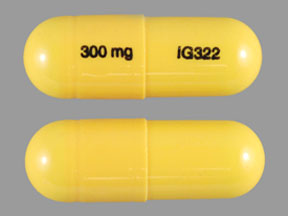
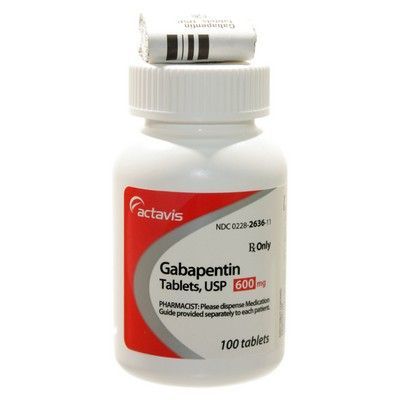
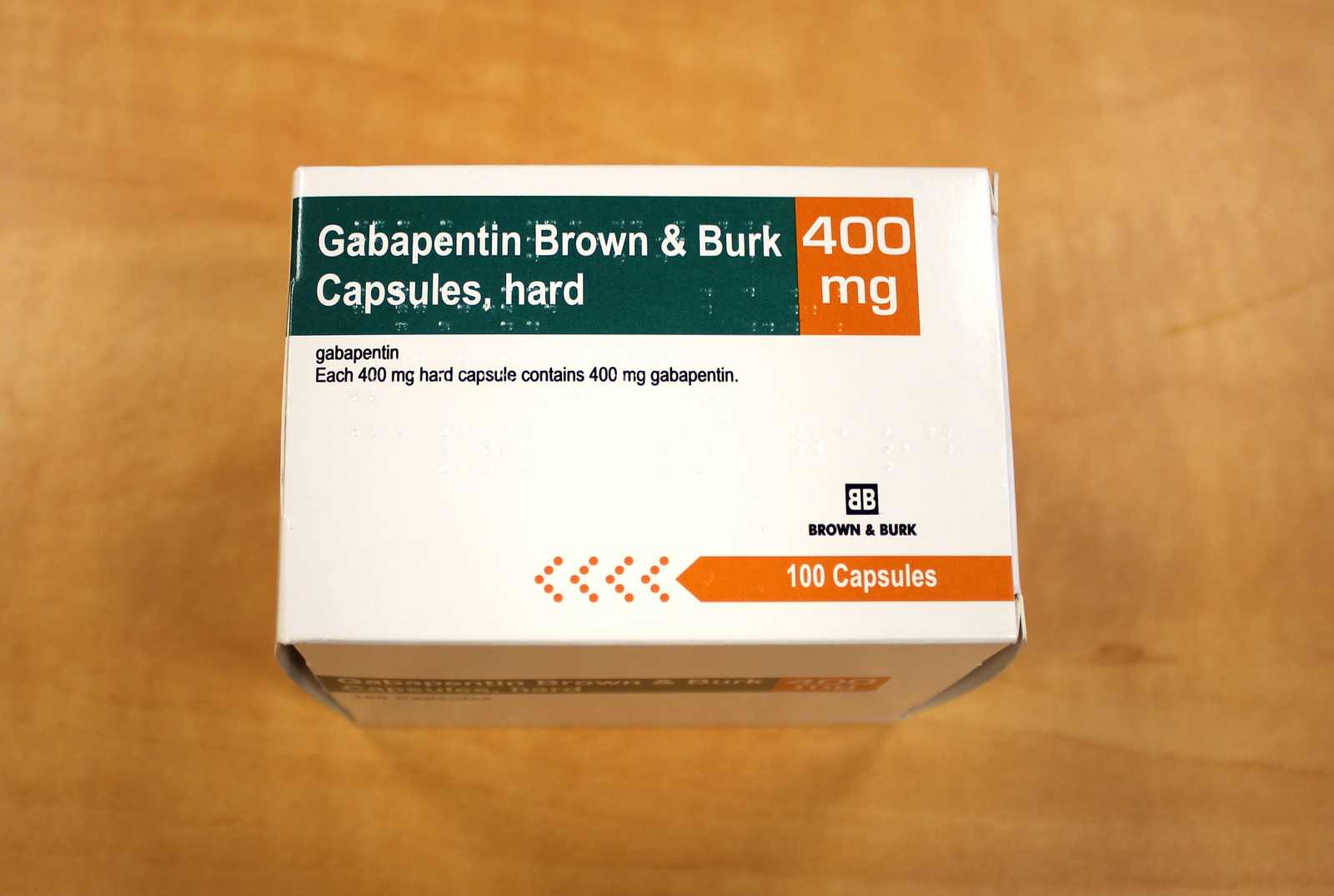
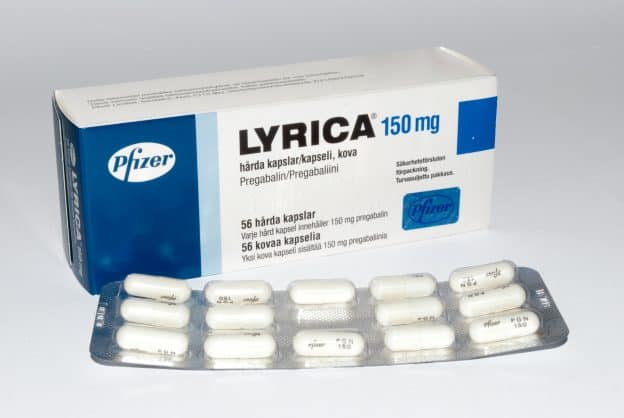
A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1,200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1,200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Gabapentin (Neurontin) is FDA approved to treat certain types of seizures and nerve pain. Opioids are approved to treat moderate to severe pain. Gabapentin is sometimes used “off-label” as an alternative to opioid medications to help manage pain. Opioids have a higher potential for dependence and addiction than gabapentin. The oral solution contains 250 millgrams of gabapentin per 5 milliliter (50 mg per mL) Neurontin or generic gabapentin. Gabapentin capsules. It’s available as 100-, 300- or 400-milligram gelatin capsules (Neurontin or generic gabapentin). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. It comes as 100, 300, or 400 mg oral capsules; 600 Like gabapentin, it's taken for epilepsy and nerve pain. It can also be taken for anxiety. But there are differences between pregabalin and gabapentin. Pregabalin can be taken less often and in different doses to gabapentin. If you need to change to pregabalin, your doctor will explain how to swap safely from gabapentin. 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose Gabapentin Capsules, USP are available containing 100 mg, 300 mg or 400 mg of gabapentin, USP, supplied as follows: 100 mg capsules: Size '3' Hard gelatin capsules with white opaque cap and white opaque body, imprinted "100 mg" in blue ink on cap and "236" in blue ink on body, filled with white to off-white powder. Capsules are supplied in Although the anticonvulsant is not considered a controlled substance, some state legislation focuses on monitoring the use of or reclassifying it. The FDA approved gabapentin in 1993 as a non-controlled substance and it has remained a non-controlled substance at the federal level. Some other states don't classify gabapentin (Neurontin) as a controlled substance, but they do keep close track of gabapentin (Neurontin) dispensing using a prescription monitoring program. If you have questions about controlled substance laws in your state, it's best to talk to a local healthcare professional. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin may cause side effects such as dizziness, drowsiness, and dizziness. It is important to follow the prescribed dosage and seek medical attention if experiencing serious side effects or changes in mood or behavior. Gabapentin is prescribed by healthcare professionals and should only be taken under medical supervision. A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg GABARONE capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |