Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 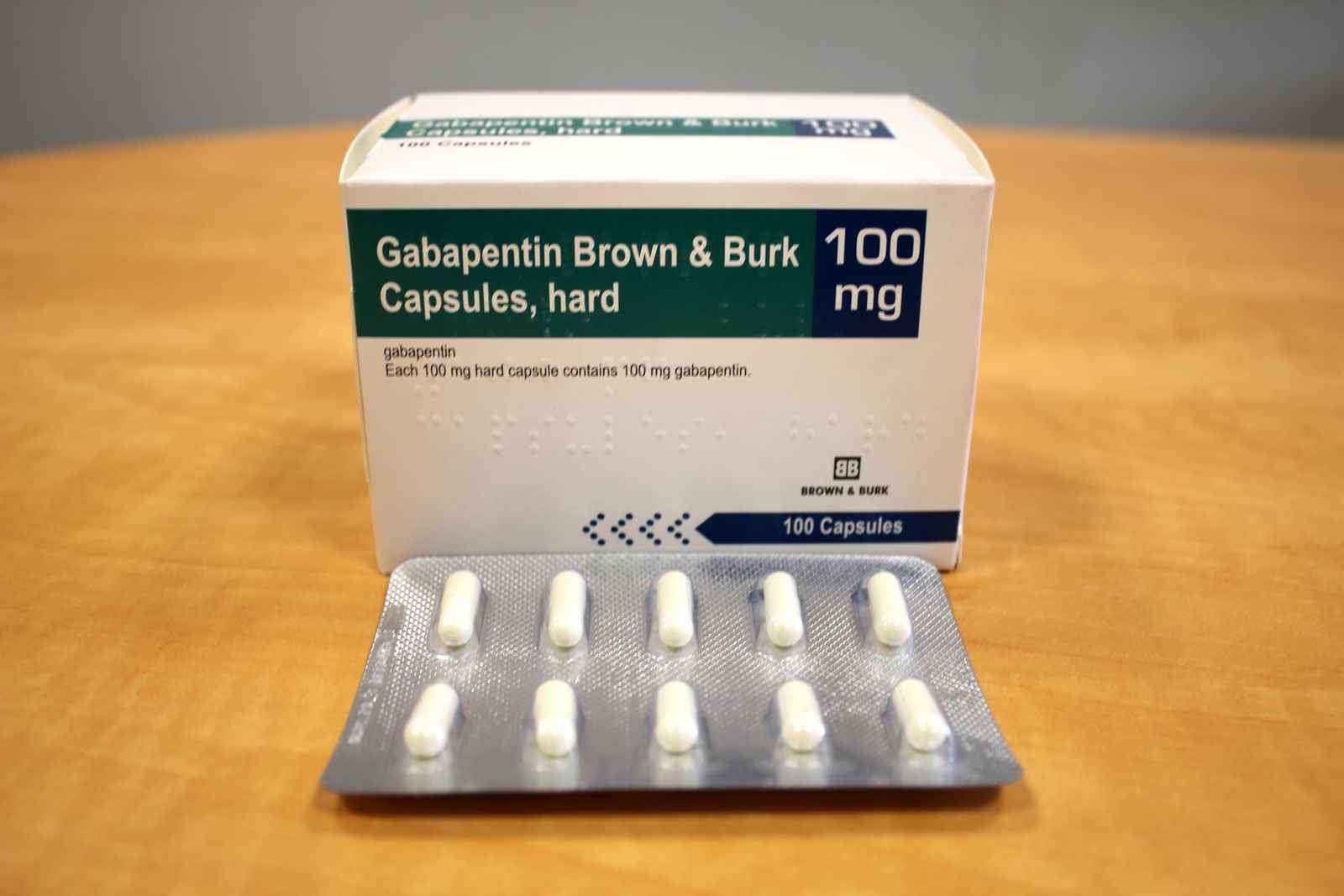 |
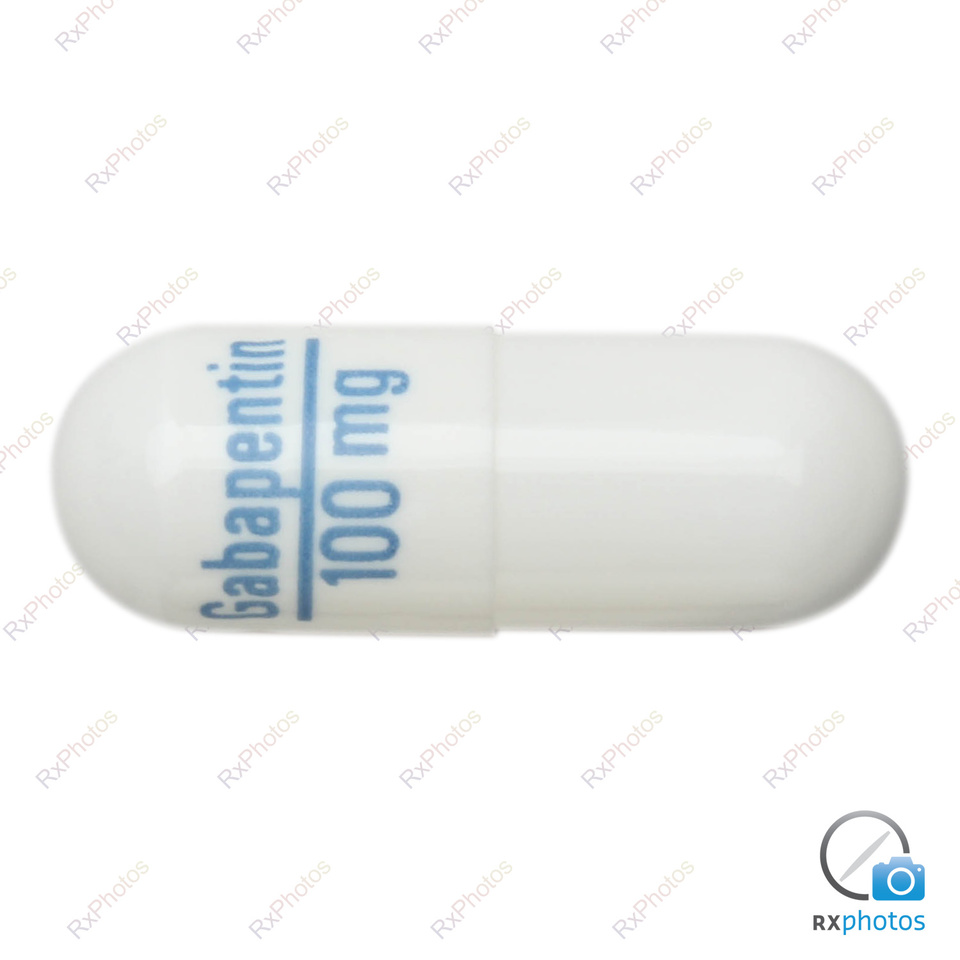
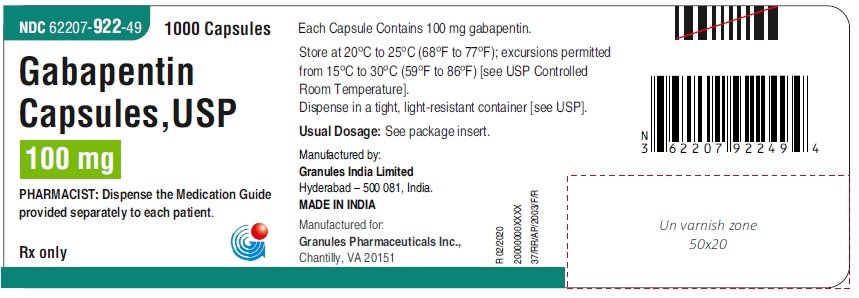
The NDC Packaged Code 67877-223-05 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Ascend Laboratories, Llc. The product's dosage form is capsule and is administered via oral form. The NDC code 63739-903 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Mckesson Corporation Dba Sky Packaging. The product's dosage form is capsule and is administered via oral form. Yes, Gabapentin with product code 72865-253 is active and included in the NDC Directory. The product was first marketed by Xlcare Pharmaceuticals, Inc. on May 10, 2021 and its listing in the NDC Directory is set to expire on December 31, 2025 if the product is not updated or renewed by the manufacturer. Gabapentin capsules are indicated for: 1 Management of postherpetic neuralgia in adults, 2 Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. The NDC code 69097-943 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Cipla Usa Inc.. The product's dosage form is capsule and is administered via oral form. List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. The NDC code 70010-227 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Granules Pharmaceuticals Inc.. The product's dosage form is tablet, film coated and is administered via oral form. Order Gabapentin 100 mg Capsule 100 Capsules by McKesson Packaging Services 63739059110 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, Gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The NDC code 65162-102 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is capsule and is administered via oral form. Complete details for NDC 67877-0222-10 Gabapentin 100 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000110 The 100 mg capsule shell contains gelatin and titanium dioxide. The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide. The 400 mg capsule shell contains gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. The imprinting ink contains FD&C Blue No. 2, propylene glycol and shellac. The NDC Packaged Code 69097-814-12 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, labeled by Cipla Usa Inc.. The product's dosage form is and is administered via form. A third study compared gabapentin 900 mg/day divided TID (N = 111) and placebo (N = 109). An additional gabapentin 1200 mg/day dosage group (N = 52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). NDC: 49483-222-00 Code: 007T. Nitro-Time 9.0 mg ER Capsule Gabapentin Capsules, USP. 100 mg / Orange, Orange / Capsules. NDC: 49483-605-00. Compare to: Neurontin Dosage: Capsule, Strength: 100 mg, Brand name equivalent: Generic of Neurontin® Capsules Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). Gabapentin is in a class of medications called anticonvulsants. Gabapentin is a human prescription drug by Ascend Laboratories, Llc. The product is distributed in 4 packages with NDC codes 67877-222-01, 67877-222-05, The study compares placebo to Gabapentin at doses of 1800 mg/day and 2400 mg/day. The results include the mean pain score over the weeks, with the baseline and subsequent scores shown for each week.* Figure 3 - image 03 100 mg Capsules (White/White colored, size 3 hard gelatin capsules with 103 printed on body of capsules containing white to off white granular powder) NDC 68071-5017-1 BOTTLES OF 100 MEDICATION GUIDE
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |