Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
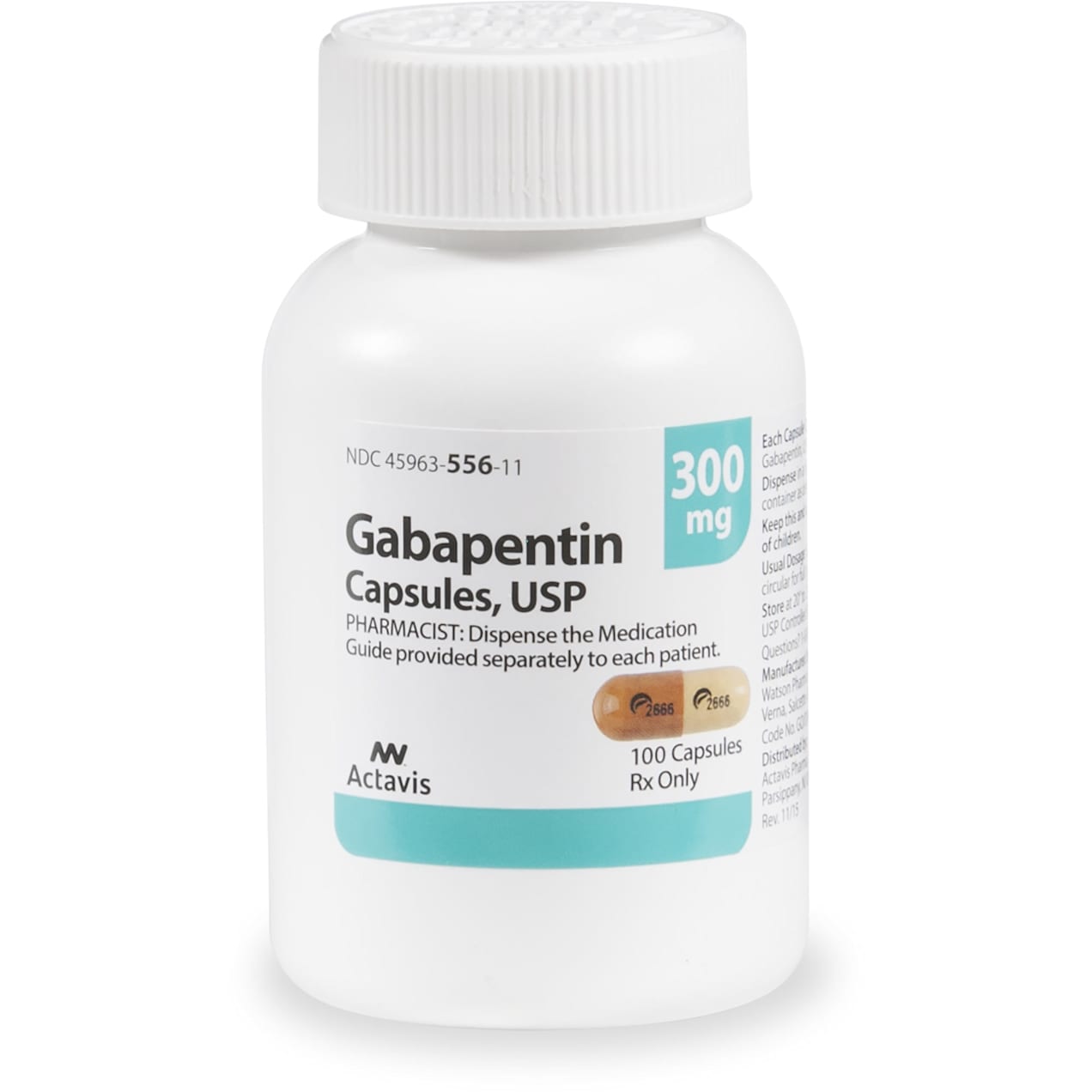
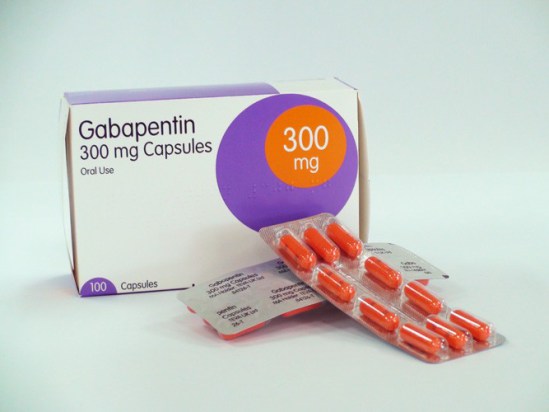
Find all the important details about this NDC Package code, including the 11-Digit NDC Billing number, billing units, wholesale price, RxNorm crosswalk, active ingredients, pharmacologic clasess, etc. Gabapentin is used with other medications to prevent and control seizures. gabapentin 300 MG Oral Capsule. notation: 310431. RXN HUMAN DRUG: US. RXN Boss From {345818} AI. RXCUI: NDC: 63629305605 76420004790 71610006030 63629305606 Gabapentin is indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and Gabapentin is a human prescription drug by Granules India Limited. The product is distributed in 3 packages with NDC codes 62207-923-43, 62207-923-47, In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical Gabapentin capsules are indicated for: 1 Management of postherpetic neuralgia in adults, 2 Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. Gabapentin is a human prescription drug by Amneal Pharmaceuticals Llc. The product is distributed in 4 packages with NDC codes 65162-102-03, 65162-102-10, Complete details for NDC 31722-0149-05 GABAPENTIN 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000130. Complete details for NDC 65162-0102-11 Gabapentin 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000130 Toggle navigation HelloPharmacist Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). Gabapentin is in a class of medications called anticonvulsants. Gabapentin is a human prescription drug by Cipla Usa Inc.. The product is distributed in 2 packages with NDC codes 69097-943-07, 69097-943-12.Gabapentin is use Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. Yes, Gabapentin with product code 0904-6666 is active and included in the NDC Directory. The product was first marketed by Major Pharmaceuticals on January 29, 2011 and its listing in the NDC Directory is set to expire on December 31, 2026 if the product is not updated or renewed by the manufacturer. NDC 70771-1861-9 in bottle of 90 tablets. Gabapentin tablets, 300 mg. Rx only. 90 tablets List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin is a human prescription drug by Mckesson Corporation Dba Sky Packaging. The product is distributed in a single package with NDC code 63739-903-10.Ga 300 mg tablets: Gabapentin 300 mg tablets are white color, oval-shaped, film coated tablets debossed with “G5” on one side and “V1” on other side. NDC 31722-091-90 (Bottle of 90) 600 mg tablets: Gabapentin 600 mg tablets are yellow color, oval-shaped, film coated tablets debossed with “G7” on one side and “V1” on other side. The NDC Packaged Code 67877-223-05 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Ascend Laboratories, Llc. The product's dosage form is capsule and is administered via oral form. Day 1: Single 300 mg dose; Day 2: 600 mg/day (i.e., 300 mg two times a day) Day 3: 900 mg/day (i.e., 300 mg three times a day) Epilepsy with Partial Onset Seizures . Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |