Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
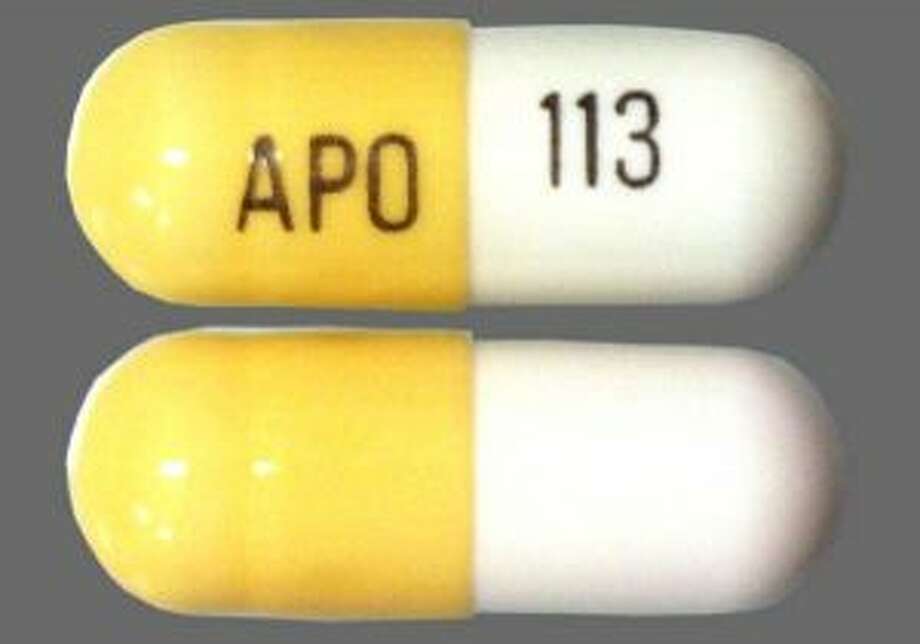
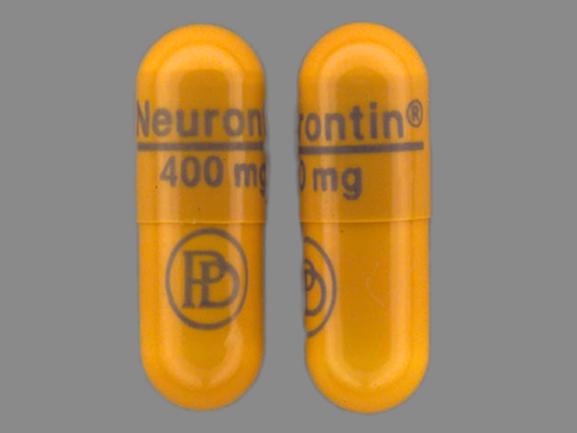
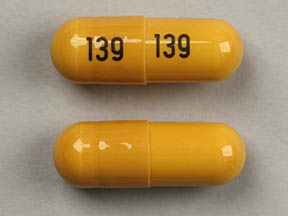
Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid During the controlled trials in patients with post-herpetic neuralgia, somnolence, and dizziness were reported at a greater rate compared to placebo in patients receiving gabapentin, in dosages up to 3600 mg per day: i.e., 21% in gabapentin-treated patients versus 5% in placebo-treated patients for somnolence and 28% in gabapentin-treated During the controlled trials in patients with post-herpetic neuralgia, somnolence, and dizziness were reported at a greater rate compared to placebo in patients receiving gabapentin, in dosages up to 3,600 mg per day: i.e., 21% in gabapentin-treated patients versus 5% in placebo-treated patients for somnolence and 28% in gabapentin-treated Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. 2024 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. In this comprehensive guide, we discuss whether or not gabapentin is considered a controlled substance in different states across the U.S., as well as its possible side effects and dangers if misused or abused. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). The number of individuals who have Gabapentin present in an accidental overdose is significant enough . to add Gabapentin dispensation into the CPRMS. Note: The Department is not changing the controlled substance scheduling of Gabapentin at this time. As such, the CPMRS look-up requirements do not apply to Gabapentin prescribing. Naloxone During the controlled trials in patients with post-herpetic neuralgia, somnolence, and dizziness were reported at a greater rate compared to placebo in patients receiving gabapentin, in dosages up to 3,600 mg per day: i.e., 21% in gabapentin-treated patients versus 5% in placebo-treated patients for somnolence and 28% in gabapentin-treated single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 On a federal level, gabapentin is not a controlled substance. However, because of increasing numbers of prescriptions and reports of misuse and harm, gabapentin is a controlled substance in some US states, with certain other states requiring gabapentin prescriptions to be reported to state databases. TABLE 1. NEURONTIN Dosage Based on Renal Function . Renal Function Total Daily Dose Regimen . Creatinine Clearance Dose Range (mg) (mL/min) (mg/day) ≥60 to 3600 300 TID 400 TID 600 TID 800 TID 1200 TID >30 to 59 400 to 1400 200 BID 300 BID 400 BID 500 BID 700 BID >15 to 29 200 to 700 200 QD 300 QD 400 QD 500 QD 700 QD 2020 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |