Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
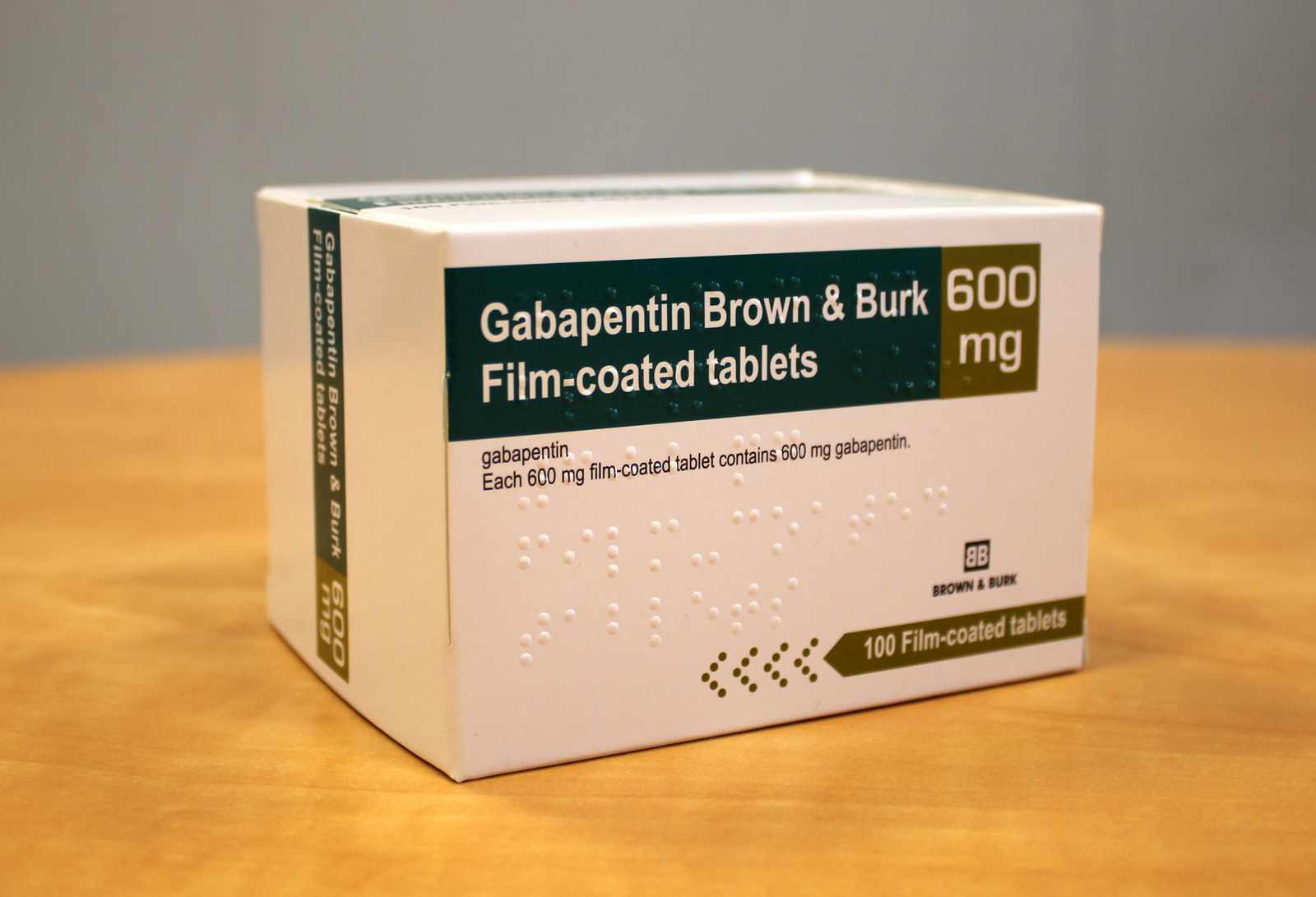
Gabapentin is indicated as monotherapy in the treatment of partial seizures with and without secondary generalization in adults and adolescents aged 12 years and above. Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. with the 600-mg dose, but caused an increase in adverse reactions. (2.1) • If the dose is not taken at the recommended time, the next dose should be taken the following day as prescribed. (2.1) PHN: The starting dose is 600 mg in the morning for 3 days, then increase to 600 mg twice daily beginning on day 4. (2.2) (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin 600 mg film-coated tablets - Patient Information Leaflet (PIL) by Aurobindo Pharma - Milpharm Ltd. Gabapentin Glenmark 600 mg film-coated tablets - Summary of Product Characteristics (SmPC) by Glenmark Pharmaceuticals Europe Ltd TEVA-GABAPENTIN tablets are supplied as follows: 600 mg tablets: White to off-white, elliptical, film-coated tablets, printed N on one side and Z600' on the other side. 800 mg tablets: White to off-white, elliptical, film-coated tablets, printed N on one side and Z800' on the other side. In a study involving healthy volunteers (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Teva Standard Teva Canada Limited 30 Novopharm Court, Toronto, Ontario Date of Revision: December 6, 2017 Canada M1B 2K9 www.tevacanada.com Submission Control No: 211039 Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Reference ID: 4584064 . This label may not be the latest approved by FDA. Each film-coated tablet contains 600 mg gabapentin. For the full list of excipients, see section 6.1. View Summary of Product Characteristics (SmPC - Gabapentin Accord 600 mg Film-coated Tablets) Gabapentin (International) In the US, Gabapentin (gabapentin systemic) is a member of the drug class gamma-aminobutyric acid analogs and is used to treat Alcohol Use Disorder, Alcohol Withdrawal, Anxiety, Back Pain, Benign Essential Tremor, Bipolar Disorder, Burning Mouth Syndrome, Carpal Tunnel Syndrome, Chronic Kidney Disease-Associated Pruritus, Chronic Pain, Cluster-Tic Syndrome, Cough Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. For oral use. Gabapentin can be given with or without food and should be swallowed whole with sufficient fluid-intake (e.g. a glass of water). Extended-release: Horizant (gabapentin enacarbil) extended release tablets: Initial dose: Day 1 to Day 3: 600 mg orally once a day Maintenance dose (Day 4 and onward): 600 mg orally twice a day Maximum dose: 1200 mg/day Comments: Gabapentin Rivopharm 600 mg film-coated tablets White to off-white, oval shaped, bevelled edged, film-coated tablet. On one side engraved “7173”, and on the other side engraved “93”. The SPC has been updated as follows: Section 4.2 – note b in Table 2 updated. Section 4.6 – Pregnancy- Risk related to gabapentin: Addition of “ Gabapentin crosses the human placenta.” Section 5.3 – Update to Teratogenesis data. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Once-daily gabapentin tablets are not interchangeable with other gabapentin products. Titrate gabapentin tablet to an 1,800 mg dose taken orally once daily with the evening meal. Gabapentin tablets should be swallowed whole. Do not split, crush, or chew the tablets. Gabapentin 600 mg film-coated tablets: White to off white coloured, film coated, elliptical shaped, lip break line on both sides, debossed with "S" and "l" separated by lip break line on one side. Tablet size is 17.30 x 9.00 mm.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |