Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
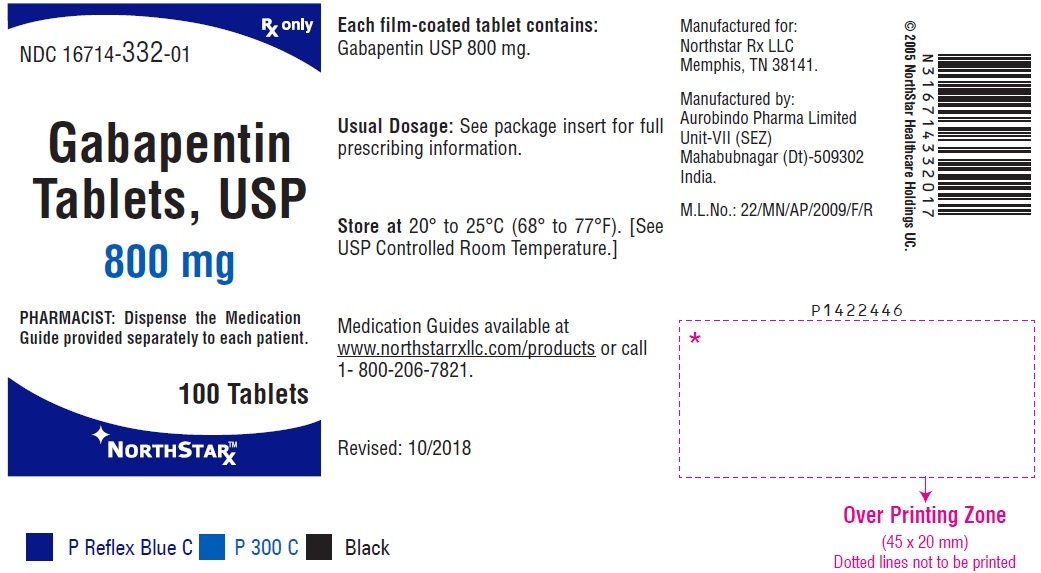 |  |
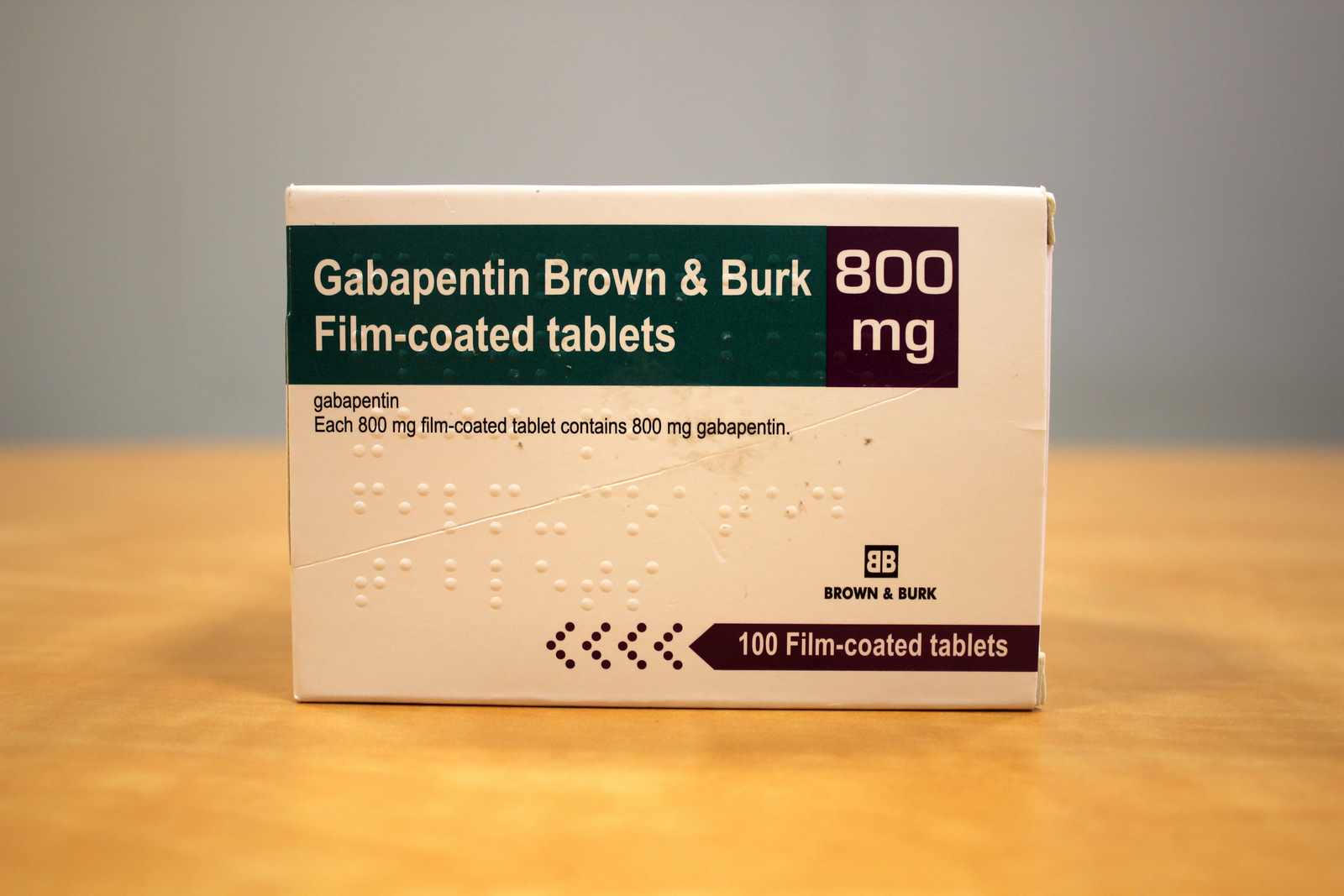
A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg capsule Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch Each Neurontin tablet contains 600 mg or 800 mg of gabapentin and the following inactive ingredients: poloxamer 407, copovidone, cornstarch, magnesium stearate, hydroxypropyl cellulose, talc, and candelilla wax. Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Gabapentin may cause side effects such as dizziness, drowsiness, and dizziness. It is important to follow the prescribed dosage and seek medical attention if experiencing serious side effects or changes in mood or behavior. Gabapentin is prescribed by healthcare professionals and should only be taken under medical supervision. Each film-coated gabapentin tablet contains 600 mg or 800 mg of gabapentin USP and the following inactive ingredients: copovidone, corn starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and talc. well tolerated. Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 years The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, What are the ingredients in gabapentin capsules? Active ingredient: gabapentin, USP. Inactive ingredients in the capsules: lactose monohydrate, corn starch, and talc. The 100 mg capsule shell contains gelatin and titanium dioxide. The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide. The active ingredient in gabapentin tablets is gabapentin, USP which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular 12.1 Mechanism of Action - The precise mechanisms by which gabapentin produces its analgesic and antiepileptic actions are unknown. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. See the end of this Medication Guide for a complete list of ingredients in gabapentin tablets. What should I tell my healthcare provider before taking gabapentin tablets? Before taking gabapentin tablets, tell your healthcare provider if you: • are pregnant or plan to become pregnant. It is not known if gabapentin can harm your unborn baby. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg capsule Each gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: anhydrous lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate, and titanium dioxide. Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc. gabapentin is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer gabapentin three times a day using 600 mg or 800 mg tablets.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |