Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
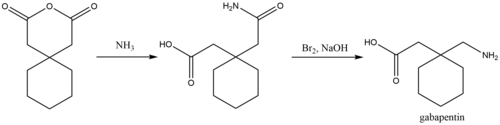
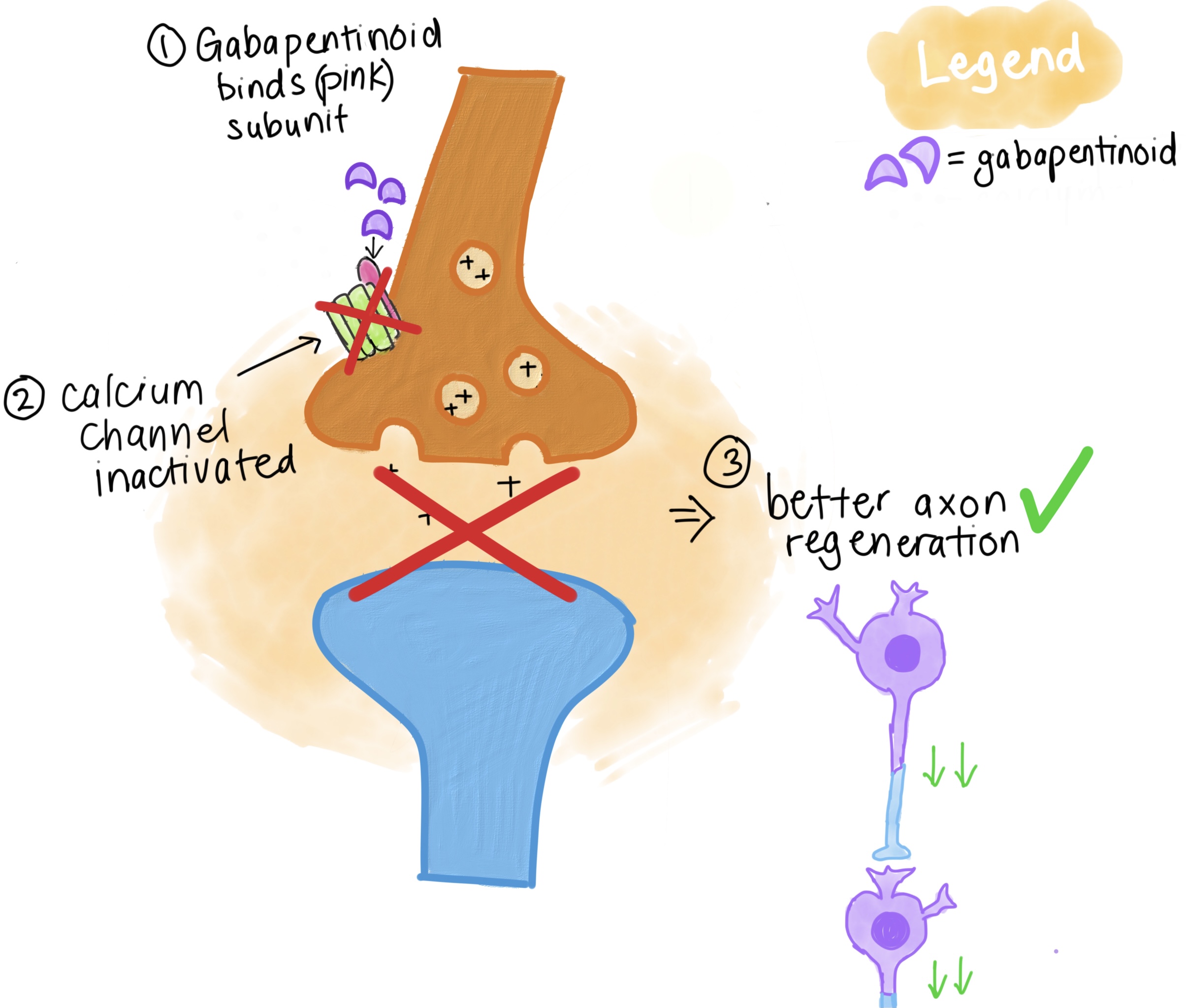
Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin is an anti-epileptic drug but its use has expanded to treat multiple other diseases including post-herpetic neuralgia, neuropathic pain, and spasticity. The mechanism of action is not fully understood but may be related to gabapentin’s action on calcium channels leading to diminution of excitatory neurotransmitters. The binding to α2δ-1 subunits inhibits nerve injury-induced trafficking of α1 pore forming units of calcium channels (particularly N-type) from cytoplasm to plasma membrane (membrane trafficking) of pre-synaptic terminals of dorsal root ganglion (DRG) neurons and dorsal horn neurons. Several mechanisms of gabapentin have been proposed after neuropathy including an inhibition of NMDA receptors, inhibition of sodium currents and reducing β4a subunit mediated VGCC trafficking (Hara and Sata 2007; Mich and Horne 2008; Yang et al. 2009). Gabapentin and pregabalin are structurally related compounds with recognized efficacy in the treatment of both epilepsy and neuropathic pain. The pharmacological mechanisms by which these agents exert their clinical effects have, until recently, remained unclear. The interaction of gabapentin and pr Mechanism of action. The precise mechanism through which gabapentin exerts its therapeutic effects is unclear. 16,17 The primary mode of action appears to be at the auxillary α2δ-1 subunit of voltage-gated calcium channels (though a low affinity for the α2δ-2 subunit has also been reported). 10,8,14 The major function of these subunits is Mechanisms of action. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. Although the exact mechanism of action with the GABA receptors is unknown, researchers know that gabapentin freely passes the blood-brain barrier and acts on neurotransmitters. Gabapentin has a cyclohexyl group to the structure of the neurotransmitter GABA as a chemical structure. Gamma-aminobutyric acid (GABA) and glutamate (GLU) play crucial roles in the control of neuropathic pain through their actions within the central nervous system (CNS). These neurotransmitters separately activate two distinct classes of receptors: ionotropic and metabotropic. Busch et al. reported that antacids reduce the bioavailability of gabapentin by ≈ 20% when given concomitantly with, or up to 2 h post, gabapentin administration . Mechanism of action. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Mechanism of action of gabapentinoids Site of action The actions of gabapentinoids are mainly at an intracellular site and require active uptake.21 They were originallydesigned as g aminobutyric acid (GABA) analogues but do not have any effects on GABA receptors. Gabapentin binds to a 2d receptors with greater affinity to the a 2d-1 subtype.22 Gabapentin is a potent activator of voltage-gated potassium channels KCNQ3 and KCNQ5, even at low nanomolar concentrations. However, this activation is unlikely to be the dominant mechanism of gabapentin's therapeutic effects. [90] Gabapentin is a structurally related to GABA that binds to voltage-gated calcium channels. It is used for various conditions such as epilepsy, neuropathic pain, restless legs syndrome, and pruritus. Mechanism of Action. The mechanism by which gabapentin exerts its analgesic action is unknown, but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce different actions responsible for pain attenuation This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. This activity also provides clinicians with the necessary skills and tools to treat various types of muscular, neurological, and psychiatric medical conditions The past 15 years has witnessed the unprecedented development of novel antiepileptic agents [1].One of the first compounds to emerge from this era was gabapentin (GBP), which was licensed for the treatment of refractory localisation-related epilepsies in the UK and Europe in 1993. Mechanism of Action The mechanism by which gabapentin exerts its analgesic action is unknown, but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). In 1 This label may not be the latest approved by FDA. Gabapentin (Neurontin) Primer Gabapentin (Trade name: Neurontin) is an anticonvulsant. It is commonly also used off-label for anxiety disorders, restless leg syndrome, and in alcohol use disorder. It is structurally similar to GABA but does not directly bind to GABA receptors. Gabapentin crosses several lipid membrane barriers via system L amino acid transporters. In vitro, gabapentin modulates the action of the GABA synthetic enzyme, glutamic acid decarboxylase (GAD) and the glutamate synthesizing enzyme, branched-chain amino acid transaminase.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |