Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
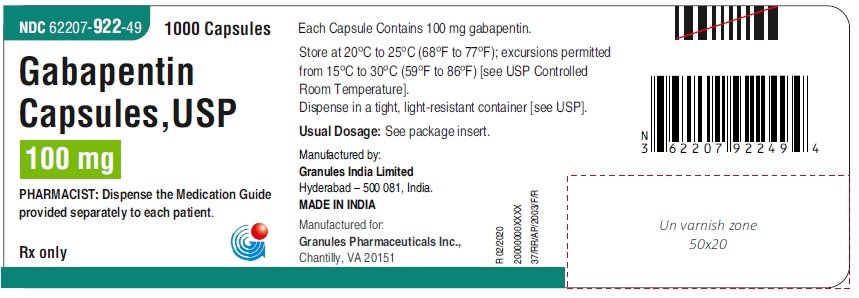
What excipients are in GABAPENTIN? Inactive ingredients, estimated generic entry dates, and list of branded drugs and generic equivalents gabapentin: 17856-0698 The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Figure 3. Proportion of Responders (patients with ≥ 50% reduction in pain score) at Endpoint: Controlled PHN Studies. Epilepsy. The effectiveness of gabapentin as adjunctive therapy (added to other antiepileptic drugs) was established in multicenter placebo-controlled, double-blind, parallel-group clinical trials in adult and pediatric patients (3 years and older) with refractory partial See the end of this Medication Guide for a complete list of ingredients in Gabapentin Oral Solution. What should I tell my healthcare provider before taking Gabapentin Oral Solution? Before taking Gabapentin Oral Solution, tell your healthcare provider if you: z are pregnant or plan to become pregnant. What are the ingredients in Gabapentin Oral Solution? Active ingredient: gabapentin 250 mg/5 mL. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. This Medication Guide has been approved by the U.S. Food and Drug Administration. Amneal Pharmaceuticals of New York LLC: Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of What are the ingredients in gabapentin capsules, USP? Active ingredient: gabapentin, USP. Inactive ingredients in the capsules: corn starch, gelatin, magnesium stearate, mannitol, sodium lauryl sulphate, talc, titanium dioxide, black edible ink which contains iron oxide black, potassium hydroxide, propylene glycol, and shellac. Gabapentin is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Amneal Pharmaceuticals Llc. The primary component is Gabapentin. Sample Package? 65162-698 National Drug Code registration, ingredients, and packaging details. Gabapentin Oral Solution, 473 ml (Xylitol Free) , RX GENERICS, Manufacturer Item #:65162069890, Patterson Item #:07-893-5342 Support Production Animal Equine Blog Resources Pivetal Close Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). Gabapentin is in a class of medications called anticonvulsants. What are the ingredients in gabapentin? Active ingredient: Gabapentin USP . Inactive ingredients in the capsules: anhydrous lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin, sodium lauryl sulfate, and titanium dioxide. The active ingredient in gabapentin oral solution is gabapentin, USP which has the chemical name 1-(Aminomethyl) cyclo hexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Pill with imprint IP 101 IP 101 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by Amneal Pharmaceuticals. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during approximately 3 days. The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral What are the ingredients in gabapentin oral solution? Active ingredient: gabapentin, USP . Inactive ingredients in the oral solution: acesulfame potassium, carboxymethylcellulose sodium, magnasweet, peppermint flavor, potassium sorbate and strawberry anise artificial flavor. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Oral solution: 250 mg per 5 mL (50 mg per mL), clear pale yellow to yellow solution. Gabapentin oral solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with gabapentin. Gabapentin is a human prescription drug by Amneal Pharmaceuticals Llc. The product is distributed in 4 packages with NDC codes 65162-102-03, 65162-102-10, For more information go to www.amneal.com. or call 1-877-835-5472. What are the ingredients in gabapentin oral solution? Active ingredient: gabapentin, USP Inactive ingredients in the oral solution: acesulfame potassium, carboxymethylcellulose sodium, magnasweet, peppermint flavor, potassium sorbate and strawberry anise artificial flavor.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |