Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
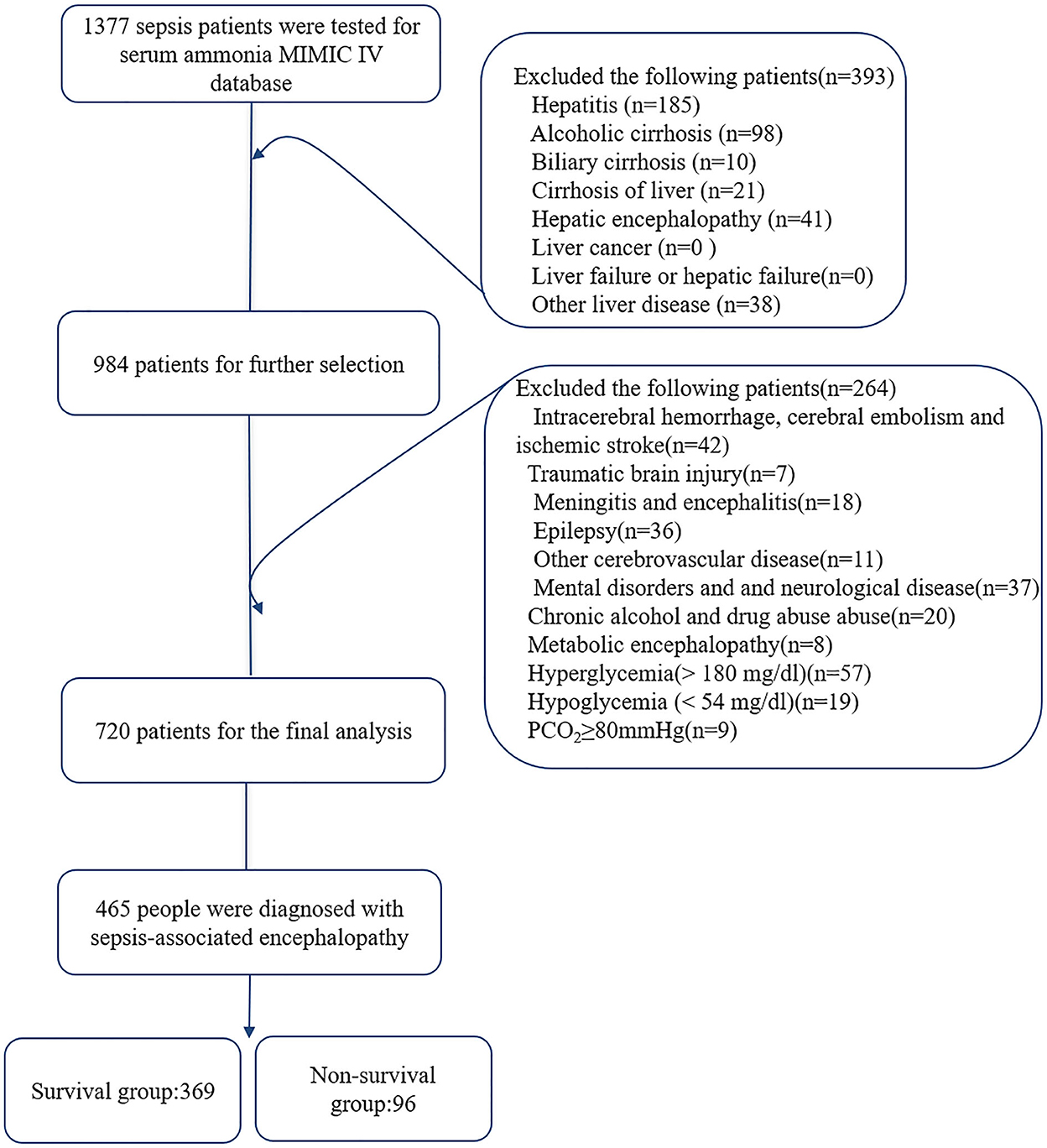
Hepatic encephalopathy represents a reversible decrease in neurological function caused by liver disease. Overall incidence of seizures in hepatic encephalopathy varies between 2% and 33%. Non-convulsive status epilepticus may be particularly common in these patients. Psychiatric disturbances manife Gabapentin-induced encephalopathy. Gabapentin-induced encephalopathy. Gabapentin-induced encephalopathy Clin Neurophysiol. 2015 Apr;126(4) :845-6. These findings are the bases of the following hypotheses: (i) when the liver fails, gut-derived GABA in plasma crosses an abnormally permeable blood-brain barrier and by mediating neural inhibition contributes to hepatic encephalopathy; (ii) an increased number of GABA receptors in the brain found in liver failure increases the sensitivity of Prescribing advise for patients with hepatic impairment. Hepatotoxicity is either dose-related or unpredictable (idiosyncratic). Drugs that cause dose-related toxicity may do so at lower doses in the presence of hepatic impairment than in individuals with normal liver function, and some drugs that produce reactions of the idiosyncratic kind do so more frequently in patients with liver disease. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is a promising intervention to reduce the burden of hepatic encephalopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is a promising inter-vention to reduce the burden of hepatic encephalopathy. Factors to consider include potential for overuse/abuse, severity of hepatic and renal impairment, and presence of hepatic encephalopathy. Chronic pain can be well managed in patients with cirrhosis; however, choice of analgesic and dosing regimen should be highly individualized and side effects carefully monitored. Gabapentin and pregabalin are safe; use cautiously in patients with hepatic encephalopathy. Topical lidocaine and diclofenac are safe. Pain is common and affects 30%-79% 1 of patients with cirrhosis. Gabapentin is a unique anticonvulsant that is used as adjunctive therapy in management of epilepsy and for neuropathic pain syndromes. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is a promising intervention to reduce the burden of hepatic encephalopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is Opioids carry the risk of precipitating hepatic encephalopathy and should generally be avoided, when possible. If clinical situation demands their use, opioid use should be limited to short-acting agents for short duration. Gabapentin and pregabalin are generally safe. Duloxetine should be avoided in hepatic impairment. Hepatic encephalopathy (HE) is a devastating complication of cirrhosis. Data are limited regarding the incidence of and risk factors for HE among contemporary patients in the context of the shifting epidemiology of cirrhosis. Gabapentin has been known to cause hyperammonaemia and toxic encephalopathy in the absence of liver failure . The paracetamol dose was low, but it is possible that it contributed through a subclinical liver inflammation, and thereby reduced liver function as seen in other UCDs [ 18 ]. Higher risk of encephalopathy was reported in patients with history of severe hepatic encephalopathy, ethanol-related hepatic failure, metabolic liver disease, greater severity of pre-transplant liver injury (defined by Child-Pugh or MELD scores) and non-elective liver transplantation[41,46,47]. Quite often, multiple co-existing risk factors Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Keywords: gabapentin, hepatotoxicity, drug-induced liver injury. Introduction Purpose: To date, only one case of asterixis associated with the use of gabapentin (GBT) has been reported. No data, instead, are available on the occurrence of asterixis related to a dementing encephalopathy during GBT therapy in the elderly. Tramadol: Tramadol undergoes more than 80% hepatic metabolism. 4 While it has been expected to provide less pain relief in cirrhotic patients due to decreased biotransformation, this has not been seen in clinical studies. 4 One study noted a significant difference in the peak concentration (C max) and peak time (T max), clearance reduced by Multiple medications are associated with an increased risk of incident hepatic encephalopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |