Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 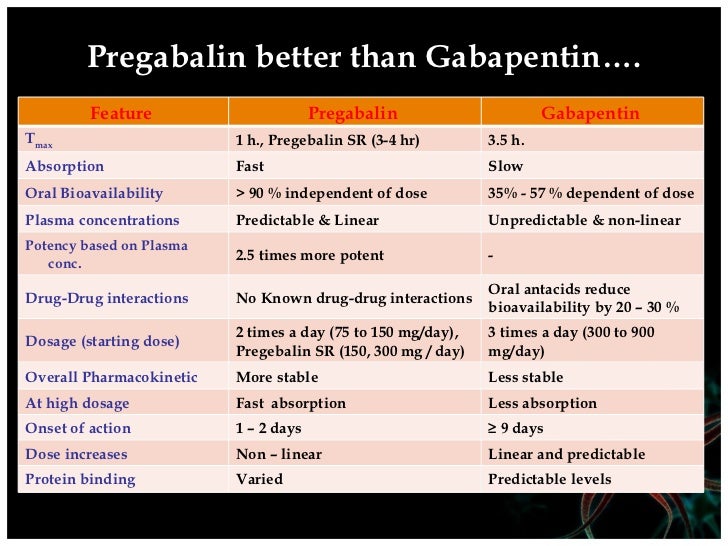 |
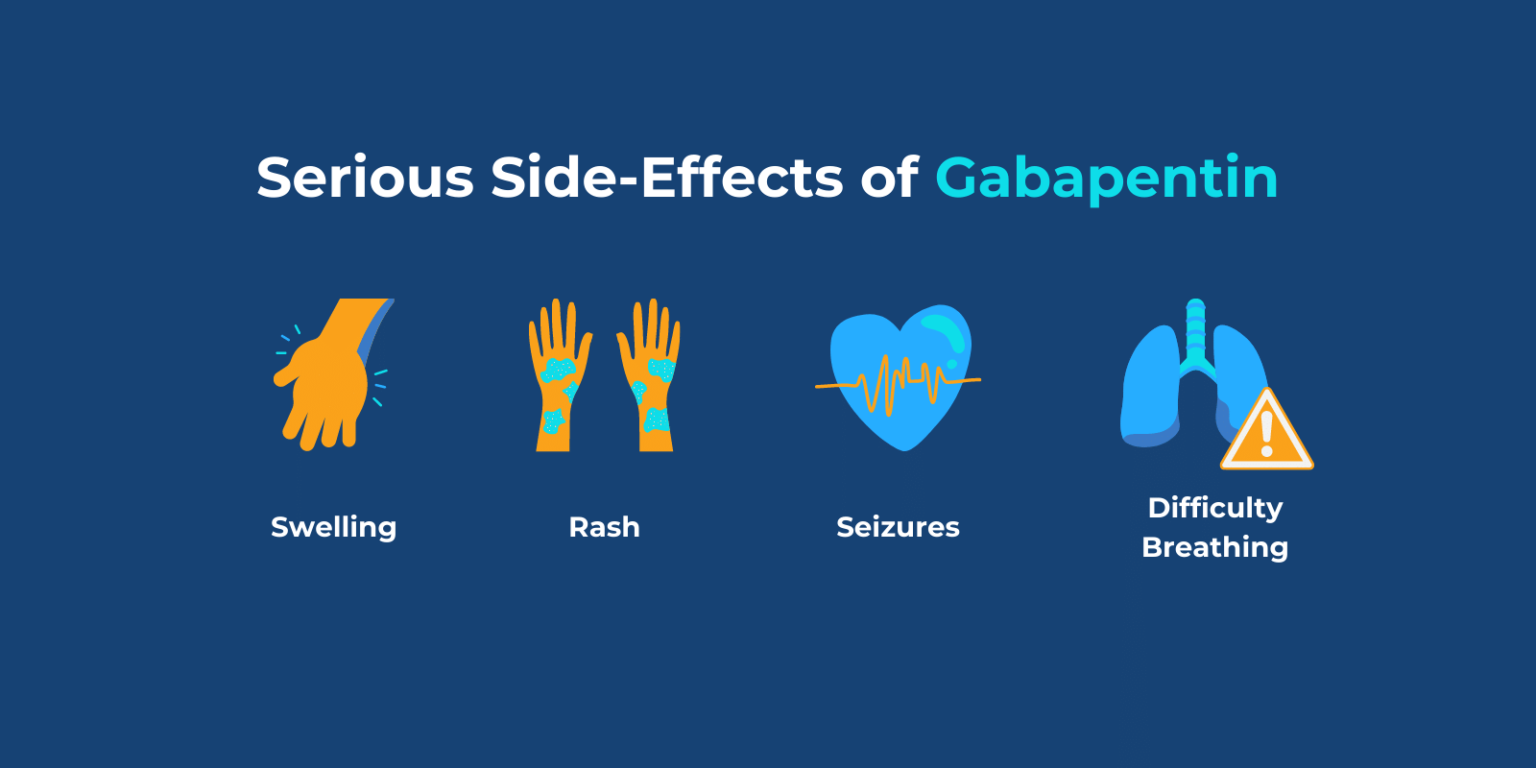
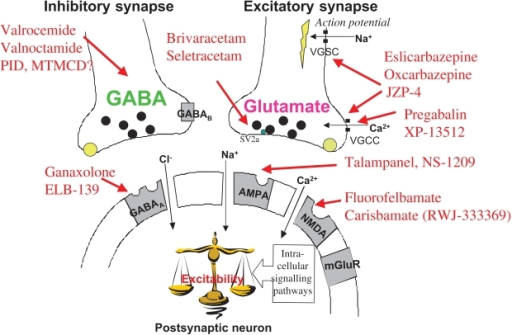
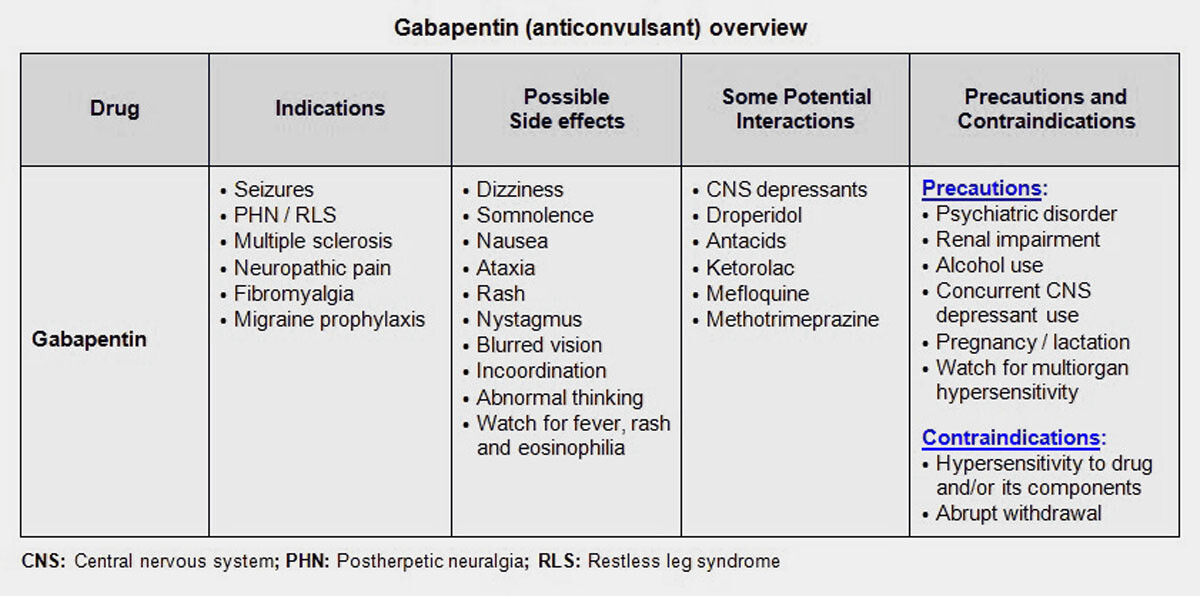
Request PDF | Gabapentin-associated hepatotoxicity | The American Journal of Gastroenterology is published by Nature Publishing Group (NPG) on behalf of the American College of Gastroenterology Hepatotoxicity is also a rare and unexpected side effect of some antiepileptic drugs. Drugs such as valproic acid, phenytoin, and felbamate, have a well-recognized association with liver toxicity. Other antiepileptic drugs, including phenobarbital, benzodiazepines, ethosuximide, and the newer generations of antiepileptic drugs, have only rarely Gabapentin, a water-soluble amino acid, is eliminated unchanged by the kidneys and there is no appreciable metabolism by the liver. However, there are a few descriptions of gabapentin-related Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Liver and renal functions were impaired by gabapentin; where hepatotoxicity was associated by an imbalance in the redox status. However, magnesium only elevated blood urea nitrogen (BUN). Histologically, acute hepatitis was seen in five cases, and chronic hepatitis was seen in one case. Gabapentin was reported to cause cholestasis in two case reports. Despite the small number of reported cases of hepatotoxicity, trazodone and gabapentin are known causes of liver injury, and clinicians should be aware of this possibility. Materials and methods: Four groups of adult male rats were included; control, gabapentin (100 mg/kg/day), Vitamin E (80 mg/kg/day), and a combination of gabapentin and Vitamin E for 90 days. Serum levels of AST, ALT, LDH, ALP, urea, and creatinine were measured in addition to malondialdehyde (MDA), and reduced glutathione (GSH) tissue levels. Hepatotoxicity. Limited data are available on the hepatotoxicity of gabapentin. In clinical trials in diabetic neuropathy and epilepsy, therapy with gabapentin was not associated with an increased frequency of serum aminotransferase elevations or liver toxicity. We report a case of serious hepatotoxicity associated with gabapentin. 3, 4 During a routine visit to a diabetes clinic, a 50 year old man complained of symptoms attributable to his peripheral diabetic neuropathy. Gabapentin lacks liver metabolism; the mechanism by which it produces liver injury is still unknown; however, there are reports of hepatotoxicity associated with its administration, so its use must be individualized for each patient. Hepatotoxicity is reported as a side effect among people who take Gabapentin (gabapentin), especially for people who are female, 50-59 old, have been taking the drug for < 1 month also take Humira, and have Rheumatoid arthritis. The study analyzes which people take Gabapentin and have Hepatotoxicity. The treatment or (Tre-Gr), which assigned as the Gabapentin (Brown & Burk UK Ltd (BBUK®) and was allowed for the free access to normal rat chow and a single daily dose by gavage of 1 mL/rat of Background. Gabapentin enacarbil (gab" a pen' tin) enacarbil (en" a kar' bil) is a prodrug of and long acting form of gabapentin. Gabapentin and its derivative gabapentin enacarbil do not appear to act upon gaba-ergic transmission, but appear to bind and perhaps inhibit presynaptic, voltage dependent calcium channels which play a role in normal and abnormal neurotransmission. Gabapentin-associated hepatotoxicity. Gabapentin-associated hepatotoxicity. Gabapentin-associated hepatotoxicity Am J Gastroenterol. 2001 Dec;96(12) :3460-2 Gabapentin has no appreciable liver metabolism and there are only rare case reports about possible hepatotoxicity. Some of these reports describe patients taking multiple drugs, so the association remains unclear. 139 , 140 , 141 , 142 Acute, symptomatic seizures or epilepsy may complicate the course of hepatic disease. Choosing the most appropriate antiepileptic drug in this setting represents a difficult challenge, as most medications are metabolized by the liver. This article focuses on the acute and chronic treatment of seizures in patients with advanced liver disease and reviews the hepatotoxic potential of specific Gabapentin is an anti-convulsant that is also used off-label to treat neuropathic pain. It is not metabolized by the liver, and there have been few reports of hepatotoxity associated with it. We present a rare case of gabapentin-induced hepatotoxicity occurring in a young male. Herein, we report a gabapentin-induced hepatocellular injury in a patient without another identifiable cause for acute liver injury. Discontinuing gabapentin resulted in rapid reversal improvement in hepatocellular injury. Keywords: gabapentin, hepatotoxicity, drug-induced liver injury. Gabapentin and other AEDs. Four patients experienced liver injury attributed to gabapentin (Table 2 and Supplemental Table 7). All were Caucasian adults with ages, ranging from 46.5 years to 75.6 years and BMI ranging from 21.3 to 39.6 kg/m2. The latency ranged from 15 days to 58 days. In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |