Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
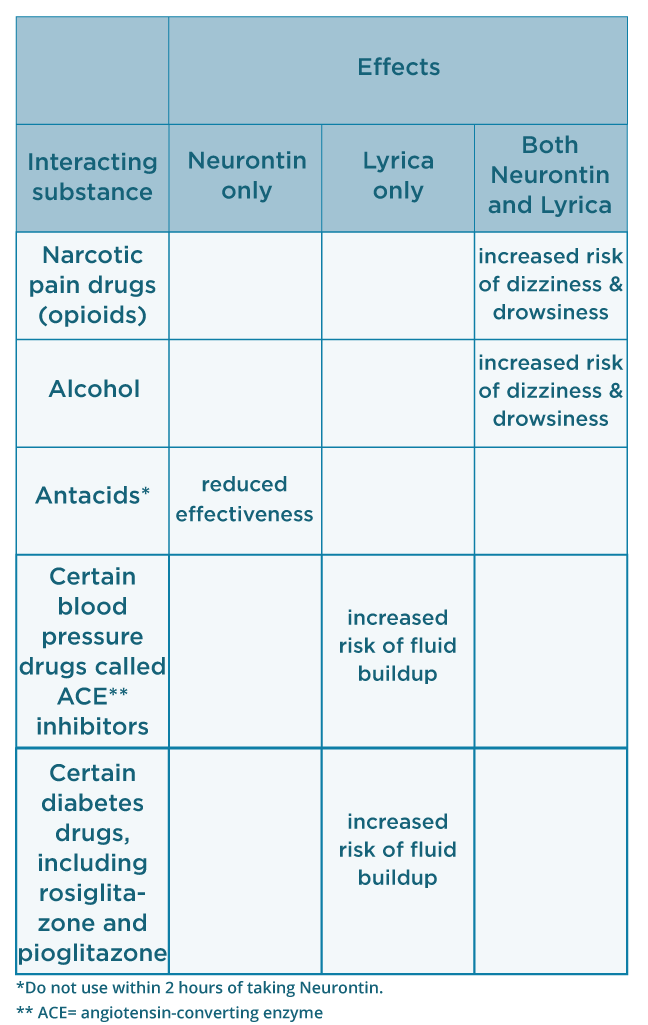
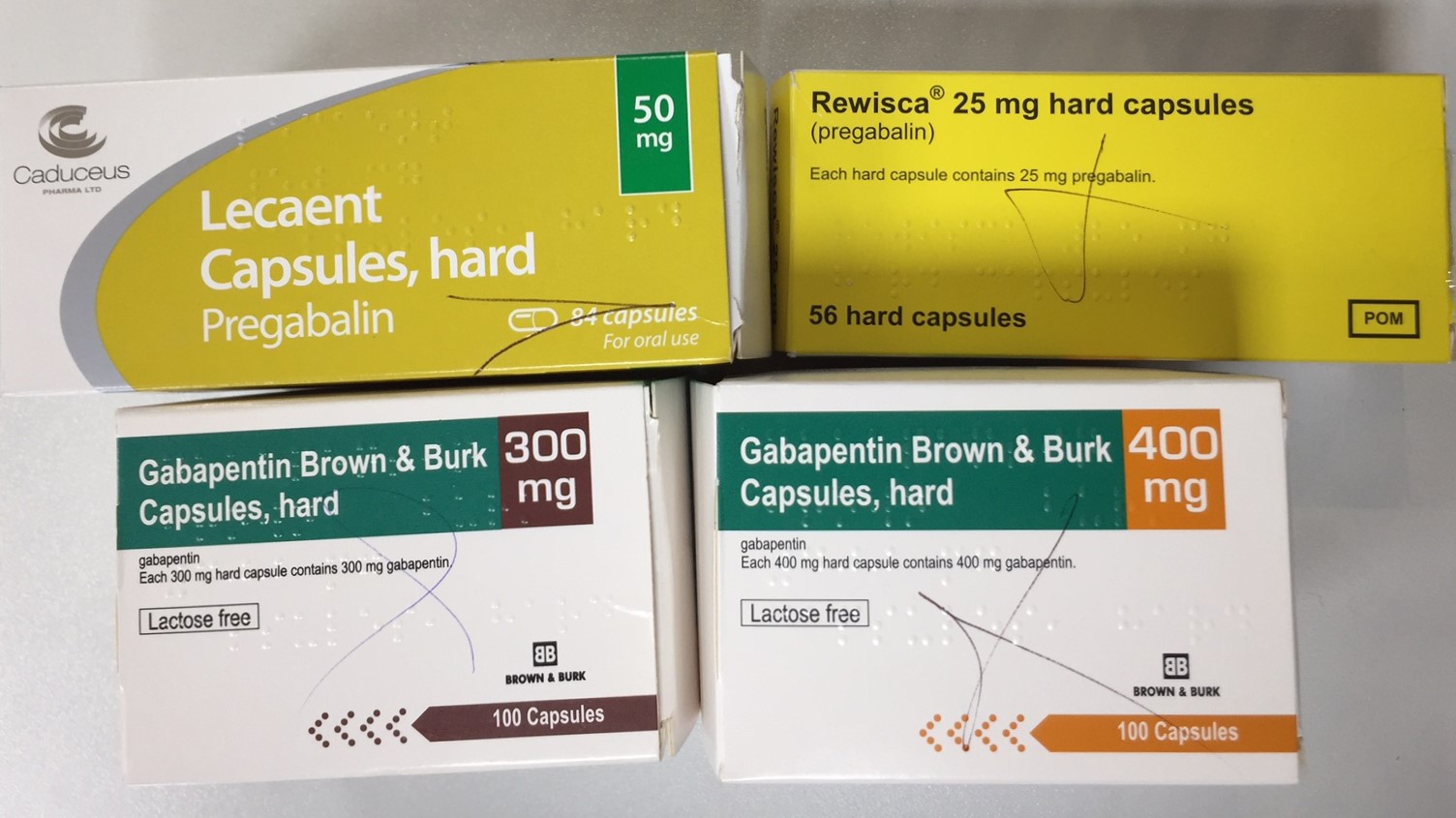
services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Background In January 2016, the Advisory Committee for the Misuse of Drugs recommended that pregabalin and gabapentin are scheduled as Sch 3 CDs within the Misuse of Drugs Gabapentin or pregabalin (Lyrica, Lyrica CR) can increase the euphoria (“high”) felt when combined with opioids, including fentanyl, codeine, oxycodone, hydrocodone, and heroin. Combining these drugs may also lead to breathing problems and life-threatening or fatal respiratory depression. Gabapentin 3 28 days 7 7 7 7 Pregabalin 3 28 days 7 7 7 7 Further information PSNC has issued a briefing on gabapentin and pregabalin’s reclassification as Controlled Drugs, which includes a set of FAQs to outline what to do in certain scenarios: ow.ly/2oD030nKQpy NHS England has issued a briefing note on Rescheduling of Gabapentin and Pregabalin is a controlled substance in every state, while gabapentin is controlled only in some states. Both medications have common side effects, such as drowsiness, dizziness, and edema. But pregabalin is more likely to cause weight gain. Pregabalin and gabapentin had been increasingly reported as drugs with a potential for misuse. They also present a risk of addiction and a potential for illegal diversion and medicinal Pregabalin and gabapentin will be reclassified as class C controlled substances in the UK from next April to reduce the growing number of deaths associated with their misuse, the government has said.1 Victoria Atkins, parliamentary under secretary for crime, safeguarding, and vulnerability, said, “Any death related to the misuse of drugs is a tragedy. Drug Interactions: A total of 270 drugs are known to interact with Gabapentin: 28 major drug interactions (148 brand and generic names) 232 moderate drug interactions (1026 brand and generic names) 10 minor drug interactions (52 brand and generic names) A total of 284 drugs are known to interact with Pregabalin: 7. Practices which are already using the EPS Schedule 2 & 3 Controlled Drugs functionality by 31 March 2019 can continue to use EPS for controlled drugs. 8. Suppliers of EPS systems will update their systems by 31 March 2019 to support the change to the classification of gabapentin and pregabalin. The timing of this will Both Lyrica and gabapentin are used as anti-epileptic medications and to treat nerve pain. But there are several differences between them. The main differences between Lyrica and gabapentin are: Lyrica is a brand name for pregabalin. Gabapentin is a generic name - brands of gabapentin include Neurontin, Gralise, and Horizant. Gabapentin and pregabalin are FDA-approved to treat some of the same conditions, including postherpetic neuralgia in adults. Both drugs are also indicated to treat partial seizures in adults and certain children with epilepsy (a seizure disorder) when taken along with other medication. Should gabapentin and pregabalin become approved additions to the existing list of controlled drugs available to physiotherapist and/or podiatrist independent prescribers, then such prescribers will again be able to prescribe them. NHS England and the professional bodies will update the professions if these changes are agreed. From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after Gabapentin and Pregabalin - Frequently Asked Questions Gabapentin and pregabalin will become Schedule 3 Controlled Drugs in April 2019 NHS Contractor Services Prescribers are legally permitted to be in possession of CDs and to supply them to others who are legally permitted to be in possession of CDs. This means that a doctor or non-medical FDA announced a new mandate that labels of gabapentin and pregabalin contain a warning about respiratory depression . ii Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin From 1 April 2019, gabapentin and pregabalin have been reclassified as controlled drugs, leading to changes in how they are prescribed. Gabapentin and pregabalin are drugs used to treat a range of symptoms caused by MS, such as nerve pain, spasticity and spasms. Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Are they controlled drugs? In short, yes they are controlled drugs but no, they do not need to be locked in a CD cabinet, recorded in a CD register or given with a witness.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |