Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
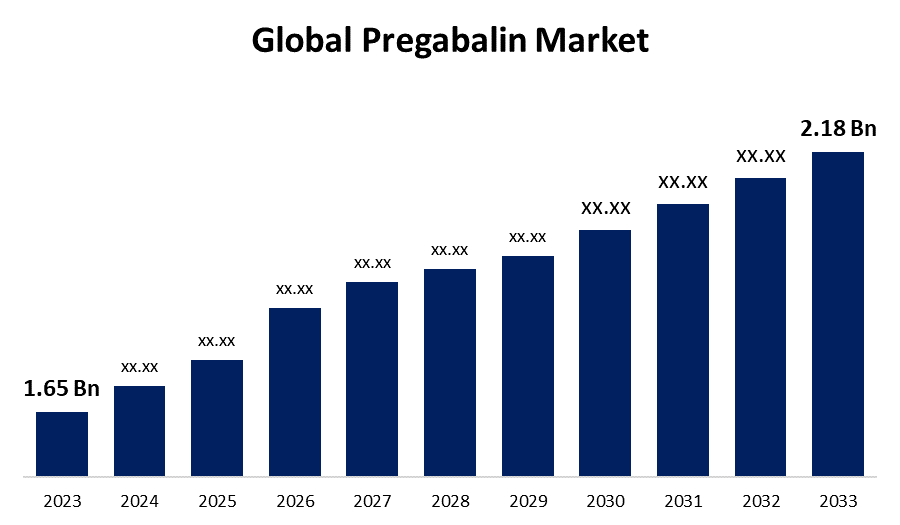
ClinicalGabapentin and pregabalin: do the bene˜ ts outweigh the harms? EE Morrison1, EA Sandilands2, DJ Webb3 Gabapentin and pregabalin prescribing in Scotland has increased substantially over recent years. Evidence suggests that prescribers may be advocating the use of these medicines off-label to avoid prescribing opioid analgesics. Gabapentin and pregabalin prescribing in Scotland has increased substantially over recent years. Evidence suggests that prescribers may be advocating the use of these medicines off-label to The evidence to support gabapentin and pregabalin use in non-neuropathic pain disorders indicates they are less effective than several other licensed non-opioid analgesics. Notably, patients may not benefit from gabapentin and pregabalin but remain at risk of adverse drug reactions. Most patients will not benefit from gabapentin or pregabalin for pain. Do not expect better pain relief from high doses. At any dose, assess for benefit or harm within 1-2 weeks; reassess often for dose reduction or deprescribing. Adverse effects increase at higher doses. The study findings suggest that both pregabalin and gabapentin can be equally used to reduce pain in lumbar radiculopathy patients who underwent TFESI. Further studies with larger sample size are needed to generalize the findings of this study. Europe PMC is an archive of life sciences journal literature. Partnerships & funding. Europe PMC is developed by EMBL-EBI with support from the Europe PMC Funders' Group, in collaboration with the National Library of Medicine (NLM), as part of the PubMed Central International archive network. Gabapentin and Pregabalin: Do the Benefits Outweigh the Harms? Journal of the Royal College of Physicians of Edinburgh, The - United Kingdom doi 10.4997/jrcpe.2017.402 (DOI: 10.4997/JRCPE.2017.402) Gabapentin and pregabalin prescribing in Scotland has increased substantially over recent years. Evidence suggests that prescribers may be advocating the use of these medicines off-label to avoid prescribing opioid analgesics. The evidence to support gabapentin and pregabalin use in non-neuropathic pain disorders indicates they are less effective than several The prescription of GP thus requires very careful and individual benefit:risk stratification , along with effectiveness and harm monitoring, and vigilant tapering/discontinuation when risks begin to outweigh benefits. From our clinician point of view, GPs are a highly useful and rather safe pharmacological armamentarium not only for neuropathic As a consequence, both gabapentin and pregabalin may soon be controlled under the Misuse of Drugs Act 1971. Prescribers should be aware of the very limited clinical evidence for use of gabapentin and pregabalin outside their licensed indications, as well as their capacity to do harm. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb. 2017 Dec;47(4):310-313 Authors: Morrison EE, Sandilands EA, Webb DJ Abstract Gabapentin and pregabalin prescribing in Scotland has increased substantially over recent years. Evidence suggests that prescribers may be advocating the use of these medicines off-label to As a consequence, both gabapentin and pregabalin may soon be controlled under the Misuse of Drugs Act 1971. Prescribers should be aware of the very limited clinical evidence for use of gabapentin and pregabalin outside their licensed indications, as well as their capacity to do harm. Gabapentin and pregabalin are approved for use in postherpetic neuralgia, while pregabalin is additionally approved to treat diabetic peripheral neuropathy, fibromyalgia and central neuropathic pain [8]. Both are also currently widely prescribed of-label for chronic low back pain [9]. Most patients will not benefit from gabapentin or pregabalin for pain. Do not expect better pain relief from high doses. At any dose, assess for benefit or harm within 1-2 weeks; reassess often for dose reduction or deprescribing. Adverse effects increase at higher doses. The evidence to support gabapentin and pregabalin use in non-neuropathic pain disorders indicates they are less effective than several other licensed non-opioid analgesics. Notably, patients may not benefit from gabapentin and pregabalin but remain at risk of adverse drug reactions. Gabapentinoids, pregabalin (LyricaTM) and gabapentin (NeurontinTM) are anticonvulsants that have been approved for chronic pain conditions such as diabetic peripheral neuropathy, postherpetic neuralgia, fibromyalgia, and are also widely prescribed off-label for chronic low back pain. Prescribers should be aware of the very limited clinical evidence for use of gabapentin and pregabalin outside their licensed indications, as well as their capacity to do harm. Gabapentin and pregabalin prescribing in Scotland has increased substantially over recent years. Evidence suggests that prescribers may be advocating the use of these medicines off-label to avoid prescribing opioid As a consequence, both gabapentin and pregabalin may soon be controlled under the Misuse of Drugs Act 1971. Prescribers should be aware of the very limited clinical evidence for use of gabapentin and pregabalin outside their licensed indications, as well as their capacity to do harm. Gabapentinoid drugs—specifically gabapentin (Neurontin) and pregabalin (Lyrica)—are increasingly being prescribed for pain because physicians and patients seek alternatives to opioids in the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |