Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
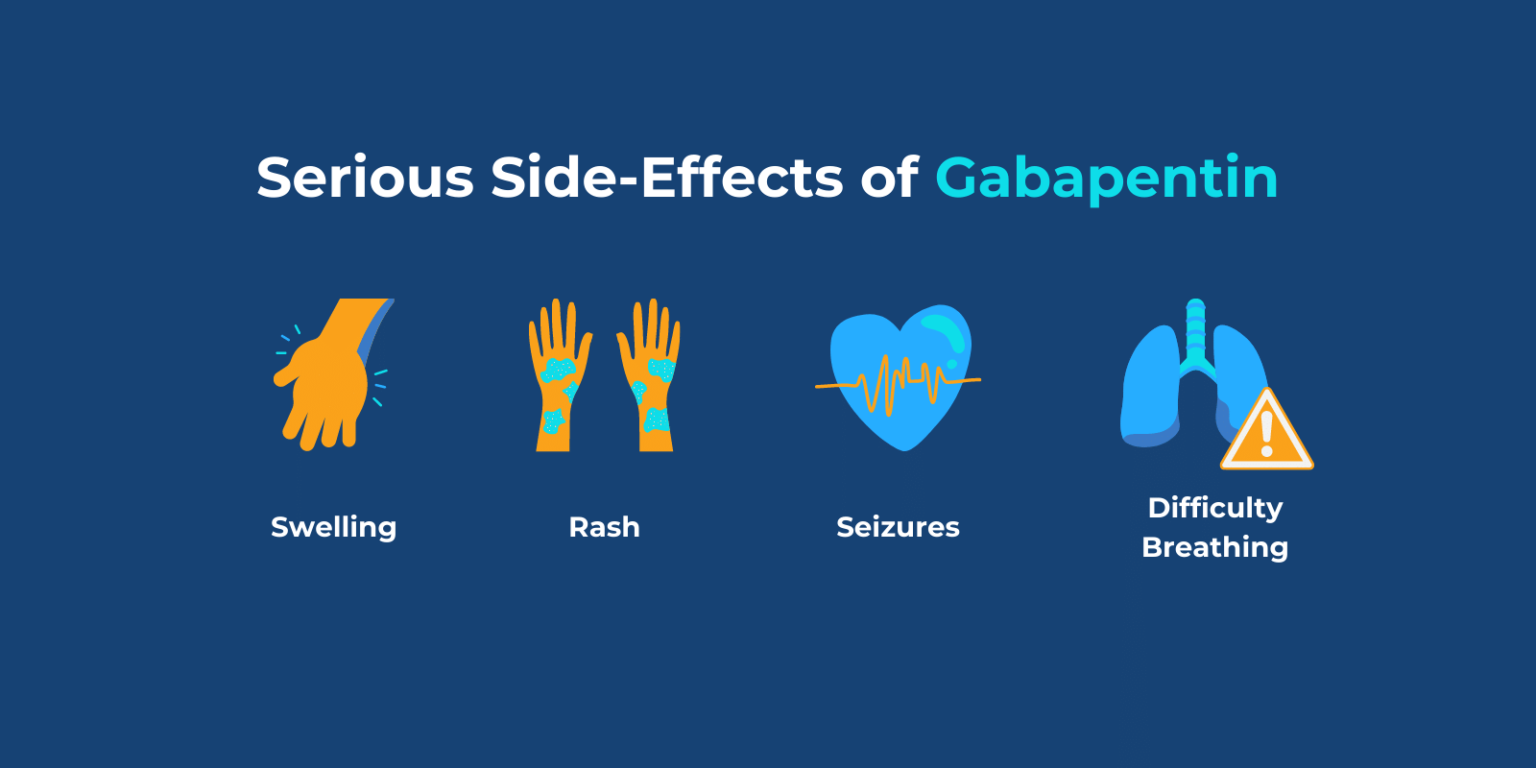
FDA is warning that serious, life-threatening, and fatal respiratory depression has been reported with the gabapentinoids, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, The last Recall Enforcement Report for Gabapentin with NDC 0904-6823 was initiated on 04-24-2023 as a Class III recall due to product mixup: one foreign tablet found in product. The latest recall number for this product is D-0570-2023 and the recall is currently terminated as of 04-30-2024 . Gabapentin is marketed as Neurontin, Gralise and Horizant and is also available as a generic. Pregabalin is sold under the brand name Lyrica and Lyrica CR. The FDA warns that use of the drugs along with opioid pain medicines or other drugs that depress the central nervous system can severely weaken breathing or cause death. ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors GRANULES PHARMACEUTICALS INC. is recalling Gabapentin Tablets USP, 600mg. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. The ongoing investigation revealed that the issue is limited to the above lot and no other lots were impacted. The last Recall Enforcement Report for Gabapentin with NDC 50228-178 was initiated on 02-17-2023 as a Class III recall due to presence of foreign tablets/capsules: pharmacist reported presence of some gabapentin tablets 800 mg comingled in gabapentin 600 mg 500 count bottles. Get an alert when a recall is issued. Do not use • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. Pregabalin (Lyrica) and gabapentin (Neurontin) are both gabapentinoids, a class of nerve medication initially developed to treat epileptic seizures. Sales of Lyrica and Neurontin tripled a decade ago, when they were touted as safer alternatives to opioids and prescribed off-label for a variety of pain conditions. The last Recall Enforcement Report for Gabapentin with NDC 70010-227 was initiated on 07-31-2024 as a Class II recall and it is currently ongoing. The last Recall Enforcement Report for Gabapentin with NDC 70010-228 was initiated on 07-31-2024 as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets The latest recall number for this product is D-0634-2024 and the recall is currently ongoing . The recall includes 7,317 devices distributed from May 11, 2018, to Sept. 5, 2019. A class I recall of the Medfusion 4000 Syringe Pump with Firmware Version 1.7.0 by Smiths Medical due to the potential for low-battery alarms to stop working. Gabapentin is being recalled due to a potential issue with empty capsules within certain batches. This isn’t a widespread recall of all gabapentin products, but rather a specific batch of 300 mg capsules manufactured by Aurobindo Pharma USA. Comparing Gabapentin with Similar Medications. Gabapentin, widely used for nerve pain and seizures, can cause memory issues in some users, such as forgetfulness or difficulty concentrating. Compared to pregabalin, which shares a similar mechanism, gabapentin’s cognitive effects are often considered milder, though this varies by individual. Mandated? Product mixup: one foreign tablet found in product. The Harvard Drug Group, LLC d/b/a Major Pharmaceuticals and Rugby Laboratories has initiated a recall for Gabapentin Tablets, 600 mg. This recall has been initiated due to a product complaint where one incorrect tablet, identified as Atorvastatin Calcium Tablets, 40 mg, was found in blister packaging for Gabapentin Tablets, USP, 600 mg. On December 19, 2019 FDA is warning that serious breathing difficulties may occur in patients using gabapentin (brand names Neurontin, Gralise, Horizant) or pregabalin (brand names Lyrica, Between 2012 and 2016, the estimated number of patients who filled a gabapentin prescription increased from 8.3 million to 13.1 million annually, and the number of patients who filled a pregabalin I was placed on Neurontin/Gabapentin in my early 20's to control seizures. After being on it for about 10 years, I went to my neurologist crying because I thought something was wrong with me. I used to have a memory so good, I could recall so many memories in my childhood and youth and had a pornographic memory. Nerve Pain, Seizure Drug Recalled. The Harvard Drug Group is pulling 3984 cartons of gabapentin tablets after a foreign tablet was found in a carton, according to the May 17, 2023, US Food and Drug Administration (FDA) Enforcement Report. Drug Recall Enforcement Report Class III voluntary initiated by Sciegen Pharmaceuticals Inc, originally initiated on 02-17-2023 for the product Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |