Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
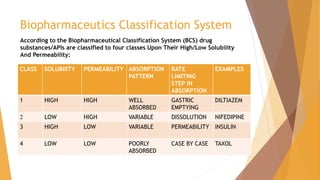
According to the biopharmaceutical classification system (BCS), Gabapentin is considered a class III drug. Its solubility is independent on the pH 21. Its absorption mainly occurs in the jejunum and the duodenum 24–26. L-amino acid transporters (LAT) are the main transporters responsible for the uptake of Gabapentin in the small intestine. BCS Class 3 Drugs List . A list of BCS Class III Drugs. Abacavir; Acyclovir; Atenolol; Gabapentin ; Glucosamine Potassium Sulphate; Lamivudine ; Levothyroxine Sodium; Gabapentin, a structural analog of gamma-aminobutyric acid (GABA), a BCS class III drug, has permeability issues that lead to bioavailability problems. To overcome these issues, gabapentin is chemically conjugated with phosphatidylcholine and loaded as nanostructured lipid carriers (NLCs), which can be targeted to Phospholipase A2. Table 4, Biopharmaceutics Classification System of antiepileptic medications - Effectiveness and Safety of Antiepileptic Medications in Patients With Epilepsy Your browsing activity is empty. Activity recording is turned off. In the BCS system, levetiracetam, gabapentin, and vigabatrin are classified as BCS class 1 drugs . These compounds are completely absorbed, with the exception of gabapentin that is about 70% absorbed in humans ( 40 ), although quite slowly. Related Searches: online bcs classification, bcs class 2 drugs list, bcs classification of drugs and its significance pdf, online bcs classification, bcs classification with examples, bcs class 3 drugs list, bcs classification fda, application of bcs classification, bcs class 4 drugs examples, potent cytotoxic drugs comes under what bcs classification, who bcs classification list. According to Biopharmaceutics Classification System (BCS), gabapentin enacarbil is classified as a BCS Class 2 compound which have a low solubility and a high permeability. Limited drug absorption due to poor solubility of BCS Class 2 drug results in poor bioavailability, ultimately hampering its therapeutic effectiveness. ICH guidance provides recommendations to support the biopharmaceutics classification of drug substances and the BCS-based biowaiver of bioequivalence studies for drug products. According to the biopharmaceutical classification system (BCS), Gabapentin is considered a class III drug. Its solubility is independent on the pH 21. Its absorption mainly occurs in the Gabapentin was encapsulated in PLGA nanoparticles as it belongs to BCS class III, with low solubility and low permeability thus make it a suitable choice for delivering via surface modified nanoparticles so as to increase bioavailability. Chitosan coated PLGA nanoparticle loaded with gabapentin were prepared by nanoprecipitation method. HorizantTM is a pro-drug of gabapentin for the treatment of moderate-to-severe primary Restless Legs Syndrome. Gabapentin enacarbil is classified as a BCS Class 2 compound (low solubility, high permeability) by the FDA. Pregabalin has been determined to be a Biopharmaceutical Classification System (BCS) Class 1 compound with high solubility/high permeability characteristics. The biowaiver request for this oral The Biopharmaceutics Classification System (BCS) classifies pharmaceutical compounds based on their aqueous solubility and intestinal permeability. gabapentin demonstrates dose-dependent The oral BA of IR gabapentin is ~60% at a dose of 900 mg/day. In the absorption of gabapentin from small intestine, an L-amino acid transport system is involved, which is saturable. This saturable transporter is reportedly responsible for less than dose-proportional increase in gabapentin exposure with increasing doses. The bioavailability Case I: anti-epileptic drugs considers BCS classification that can have a significant effect on absorption. BCS class II (carbamazepine, lamotrigine and phenytoin) and BCS class III 2. BCS Classification The BCS classifies drugs into four categories: 2.1. Examples of Drugs in Each BCS Class 2.1.1. BCS Class I: High Solubility, High Permeability Drugs that dissolve readily in aqueous media and are easily absorbed through the gastrointestinal (GI) tract.Examples Abstract. The biopharmaceutical classification system (BCS) classifies compounds based on their solubility and permeability. Regulatory agencies and health organizations have utilized this classification system to allow dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds. Download scientific diagram | The structural formulae of GABA (A) and gabapentin (B). from publication: Gabapentin: A pharmacotherapeutic panacea | Gabapentin (GBP) is a second generation per day (600 mg TID). SOLZIRA™ (Gabapentin Enacarbil) is submitted as a new molecular entity, which is a transported prodrug of gabapentin designed and engineered to be stable in gastrointestinal contents and to be actively absorbed after oral dosing. Gabapentin Enacarbil converts to gabapentin rapidly by non-specific carboxylesterase The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |