Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
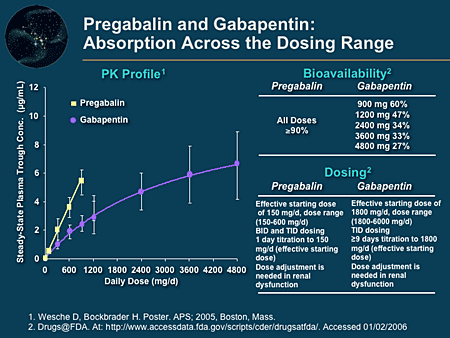
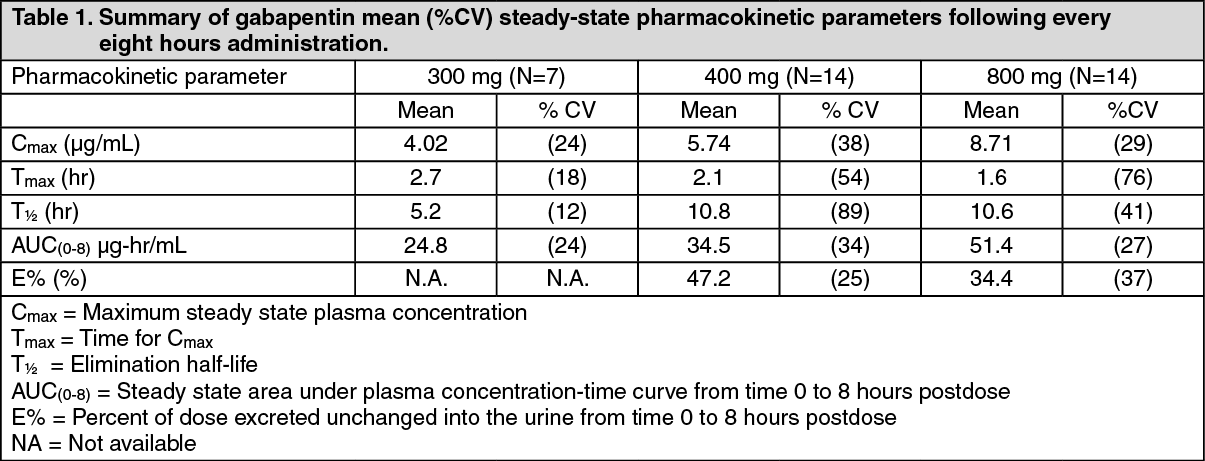
The bioavailability is also slightly enhanced with food. Gabapentin is weird in that the bioavailability goes down almost linearly with increasing dose -- so whether you're taking 150mg (about when it starts to decline) or 600mg, nearly the same amount of the drug is absorbed. This is because of the above mentioned transporter saturation. Gabapentin is a drug which is absorbed (and has a bioavailability) that is inverse to the dose taken. Some preliminary research yields this: 'Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Patients (N=12) were randomized to 3 different treatment sequences, each receiving 3 doses: a 626 mg gabapentin-equivalent dose of Horizant® with food, a 626 mg gabapentin-equivalent dose of Horizant® without food, and a 600 mg dose of conventional gabapentin without food. Patients had a 7-day washout period between doses. The purpose of this research was to establish an in vitro dissolution testing method to predict the oral pharmacokinetic (PK) profiles and food effects of gabapentin enacarbil formulated as wax matrix extended-release (ER) tablets in humans. Opening and mixing the contents of gabapentin capsules does not significantly impair drug absorption. This may be a viable administration option for patients who are unable to swallow intact capsules. Dietary macronutrient composition (i.e., protein) may favorably influence gabapentin oral absorptio Bioavailability of gabapentin is approximately 60%, 47%, 34%, 33%, and 27% following 900, 1200, 2400, 3600, and 4800 mg/day given in 3 divided doses, respectively. Food has only a slight effect on the rate and extent of absorption of gabapentin (14% increase in AUC and Cmax). Mean (standard deviation) bioavailability (based on urinary recovery) of gabapentin from gabapentin enacarbil was 42.0 (6.1)% (fasted), 64.3 (13.2)% (low-fat meal), 64.9 (16.9)% (moderate-fat meal), and 76.1 (14.4)% (high-fat meal). describe the interindividual variability in the bioavailability of gabapentin after gabapentin enacarbil administration in healthy subjects. Methods: Gabapentin pharmacokinetic (PK) parameters after an oral dose of gabapentin enacarbil 1200 mg (2 600-mg tablets) were compared across 6 phase I studies in healthy adults (n = 12 per study). The distribution of bioavailability values was assessed Antacid (Maalox®) containing magnesium and aluminum hydroxides reduced the mean bioavailability of gabapentin (N=16) by about 20%. This decrease in bioavailability was about 10% when gabapentin was administered 2 hours after Maalox. Probenecid. Probenecid is a blocker of renal tubular secretion. Mean (standard deviation) bioavailability (based on urinary recovery) of gabapentin from gabapentin enacarbil was 42.0 (6.1)% (fasted), 64.3 (13.2)% (low-fat meal), 64.9 (16.9)% (moderate-fat Absorption: Increasing gabapentin's dosage leads to reduced bioavailability; for example, daily doses of 900 mg, 1200 mg, 2400 mg, 3600 mg, and 4800 mg yield bioavailability of approximately 60%, 47%, 34%, 33%, and 27%, respectively. The impact of food is minor, resulting in a 14% increase in area under the curve (AUC) and Cmax. Bioavailability of gabapentin is approximately 60%, 47%, 34%, 33%, and 27% following 900, 1200, 2400, 3600, and 4800 mg/day given in 3 divided doses, respectively. Food has only a The olive oil is mostly unsaturated fat, making it an excellent choice for increasing absorption of Gabapentin. You need to take it with a meal to initiate the breaking down of the Gabapentin. You can't take the Gabapentin all at once, because the bioavailability decreases with higher amounts taken at once due to saturation of transporters. Oral Bioavailability Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Bioavailability of gabapentin is approximately 60%, 47%, 34%, 33%, and 27% following 900, 1200, 2400, 3600, and 4800 mg/day given in 3 divided doses, respectively. The most common AEs and their offers the potential for increased bioavailability of frequencies during each dosing period are presented in gabapentin, or maintaining the same exposure while Table IV. Subjects reported a total of 16 AEs during decreasing the dose frequency. Gabapentin (GBP) is a non-metabolized antiepileptic drug that is eliminated by renal excretion and displays saturable, dose dependent absorption. The recommended dosing schedule for GBP is t.i.d. At large daily doses, oral bioavailability (F) may be improved by giving the daily dose more frequently. Peak plasma concentrations are seen within an hour as compared to 3 hours with gabapentin. 12 Oral bioavailability for pregabalin is more than 90% as compared to 30–60% for gabapentin. These differences can be explained by the mechanism of absorption. Antacid (Maalox ®) containing magnesium and aluminum hydroxides reduced the mean bioavailability of gabapentin (N=16) by about 20%. This decrease in bioavailability was about 10% when gabapentin was administered 2 hours after Maalox. Probenecid. Probenecid is a blocker of renal tubular secretion. Gabapentin bioavailability is less than dose-proportional; however, the amount of drug absorbed increases with increasing dose. Food has no effect on the pharmacokinetics of gabapentin. Gabapentin is effective as monotherapy for partial seizures (Beydoun 1999).However, although few studies have compared the effectiveness of gabapentin with other AEDs in the management of partial epilepsy, available evidence suggests that monotherapy with gabapentin is less effective than traditional agents such as carbamazepine and the newer antiepileptic drug lamotrigine (Marson et al. 2007).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |