Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
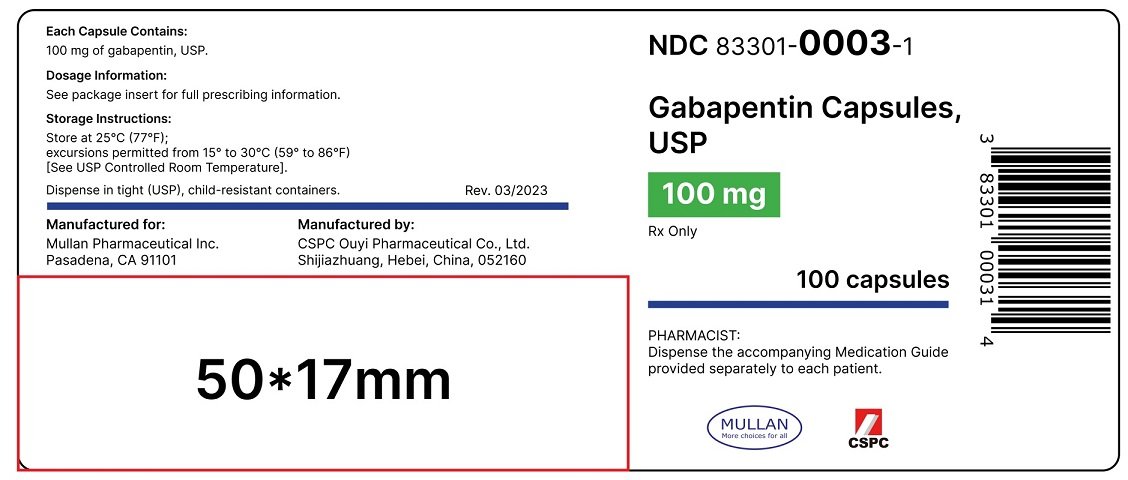
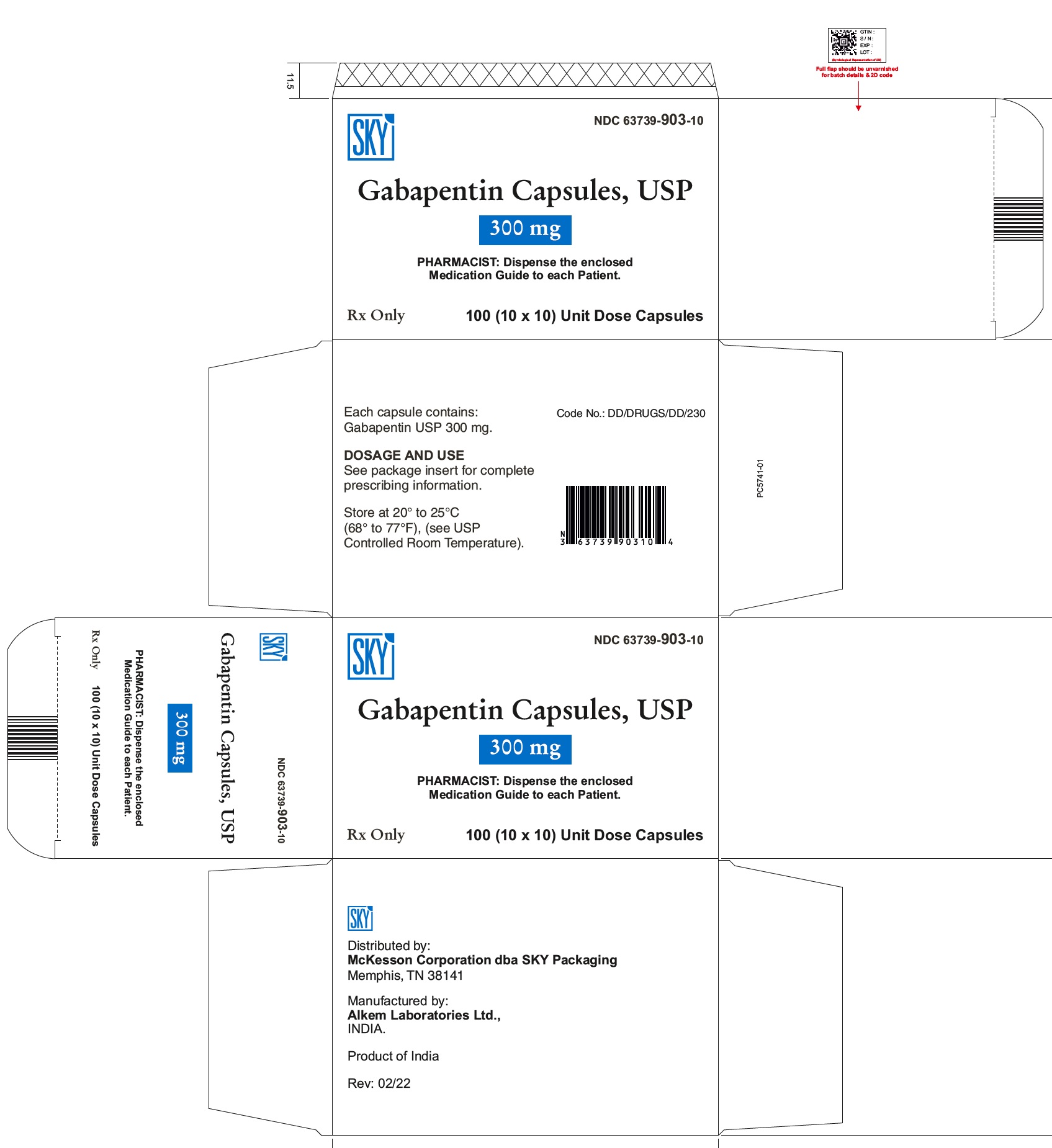
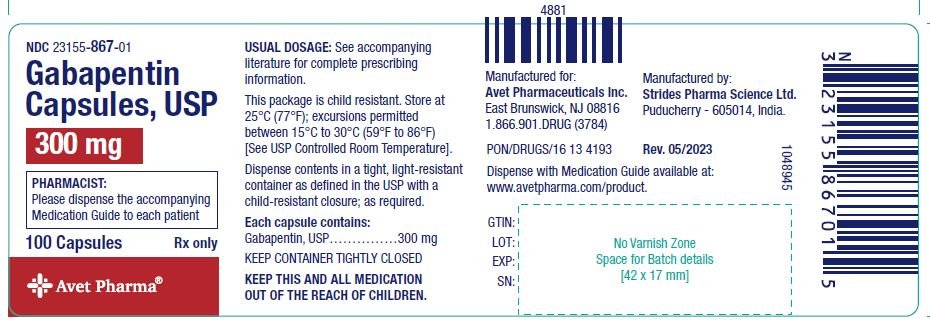
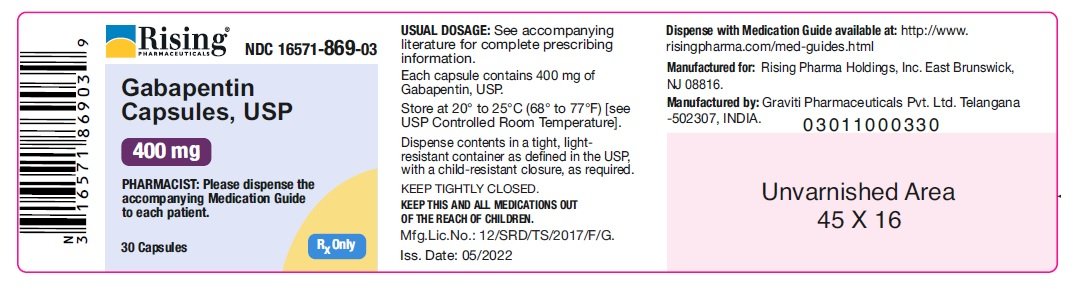
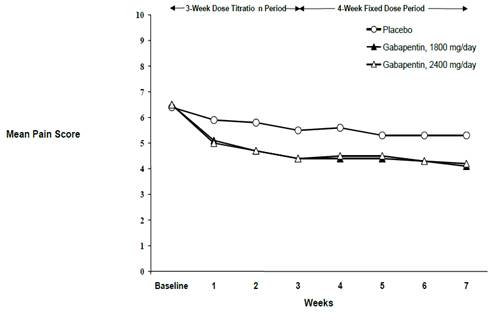
NEURONTIN capsules should be swallowed whole with water. Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Reference ID: 4168942 Gabapentin Capsules package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 GABAPENTIN- gabapentin capsule ScieGen Pharmaceuticals, Inc.-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. GABAPENTIN capsules, for oral use GABAPENTIN tablets, for oral use Initial U.S. Approval: 1993 Gabapentin: Package Insert / Prescribing Info Play pronunciation. Package insert / product label Dosage form: capsule Each gabapentin capsule contains 100 mg, 300 Administer gabapentin capsules three times a day using 300 mg or 400 mg capsules. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 years. The active ingredient in gabapentin capsules is gabapentin, which has the chemical name 1-(aminomethyl) cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. Administer gabapentin capsules orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin capsules dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. See package insert . for full prescribing information. STORAGE AND HANDLING. Store at 20 to 25 C (68 to 77 F); excursions Gabapentin capsules, USP are given Do not stop taking Gabapentin Capsules without first talking to your healthcare provider. Stopping Gabapentin Capsules suddenly can cause serious problems. Gabapentin Capsules can cause serious side effects including: 1.Suicidal Thoughts. Like other antiepileptic drugs, Gabapentin Capsules may cause suicidal thoughts or actions in a very small indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of somnolence caused by NEURONTIN, can be imperfect. The duration of . GABAPENTIN- gabapentin capsule Cipla USA Inc.-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin, USP is a white to off-white crystalline powder. It is freely soluble in water and in alkaline and acidic solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is −1.25. Gabapentin capsules are indicated for: • Management of postherpetic neuralgia in adults • Adjunctive therapy in the treatment of partial onset seizures, with and Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg NEURONTIN capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |