Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
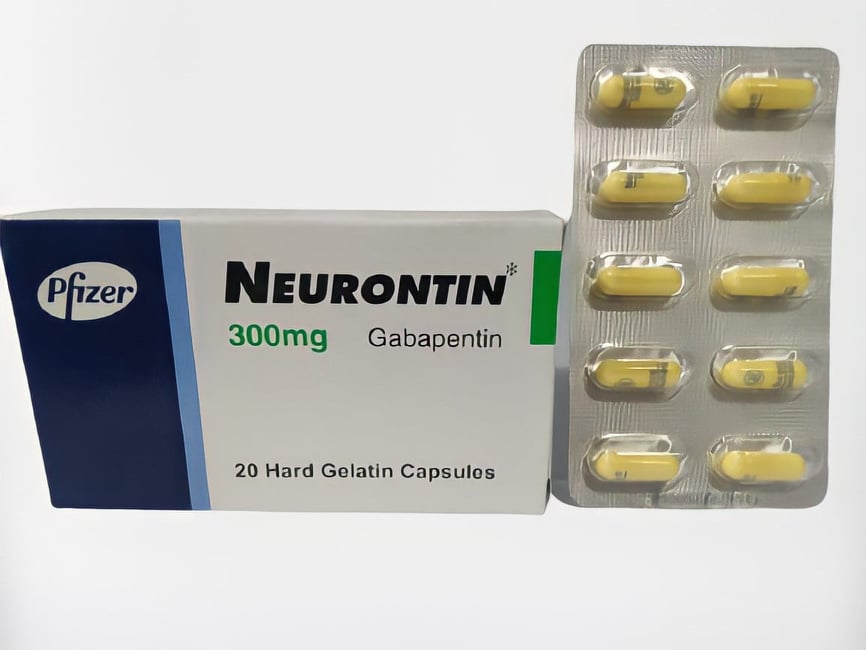
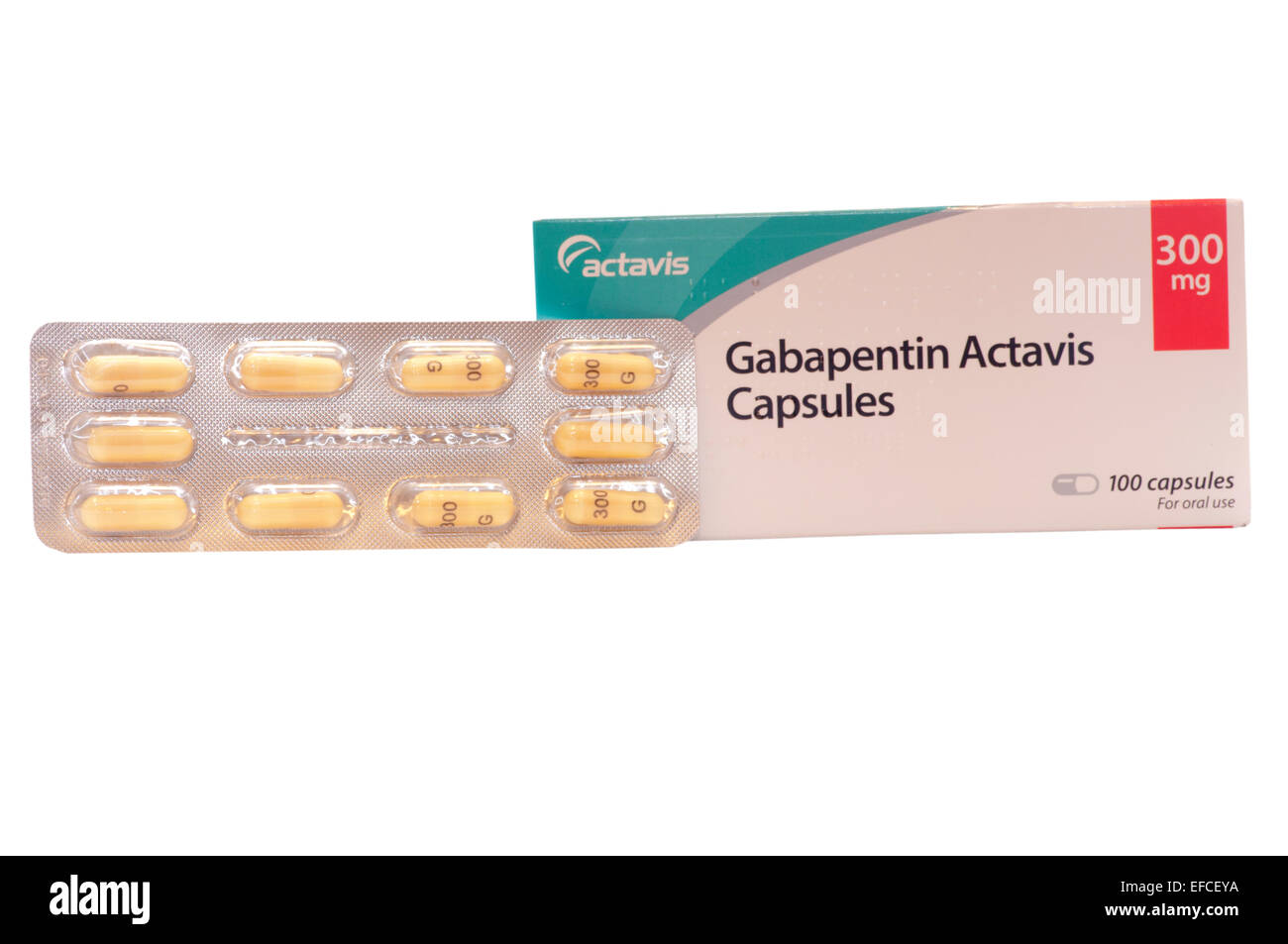
2.1.1 Ligand preparation The chemical structures of the gabapentin, phosphatidylcholine, gabapentin- phosphatidylcholine conjugate, and the oleyloxyethyl phosphorylcholine (inhibitor of phospholipase-2) were generated and optimized using ChemSketch (Advanced Chemistry Development, Inc. 8 King Street East, Suite 107, Each gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: anhydrous lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate, and titanium dioxide. Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. [0015] The present invention provides a novel process for the preparation of gabapentin (I), comprising the steps of: (a) reacting diimide VI with an alkali to produce CDMA (IV) and (b) converting CDMA (IV) to gabapentin (I). Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided The invention provides a gabapentin capsule preparation, which is a capsule directly filled with gabapentin bulk drug; the particle size distribution of the gabapentin raw material medicine is 50-70 meshes. The invention relates to a gabapentin capsule and a preparation method thereof. The gabapentin capsule comprises the following components: 150-300 g of gabapentin, 100-600 g of low substituted Check Solubility: Gabapentin is water-soluble, meaning it can be dissolved in water. However, ensure that the inactive ingredients in the capsule don’t pose any issues when dissolved. Preparation Method: Open the Gabapentin capsule and pour the contents into a small amount of water (about 5-10 mL). The ethanedisulfonic acid gabapentin pattern (bottom) indicates a mixture of gabapentin form 1, gabapentin monohydrate and the ethanedisulfonic acid gabapentin salt is present. The bottom diffractogram from gabapentin and ethanedisulfonic acid has peaks including, but not limited to, 6.01, 7.93, 15.01, 16.95, 19.75, 20.27, 21.27, and 23.09 (A) Gabapentin standard solution in mobile phase (2.5 mg/mL); (B) gabapentin suspension prepared form bulk drug using Oral Mix vehicle and submitted to sample preparation for HPLC injection (100 mg/mL suspension, 2.5 mg/mL after sample preparation); (C) gabapentin suspension prepared form bulk drug using Oral Mix SF and submitted to sample preparation for HPLC injection (100 mg/mL suspension Method of preparation of tablets was evaluated for Gaba pharmacokinetics in rabbits in comparison to Gaba-sacch PM tablet and Gaba commercial oral capsules. This formulation had enhanced in Gabapentin capsules packed into PVC/PVdC/Aluminium blister pack have been shown to be physically and chemically stable for 36 months when stored at 25°C/50%±5%RH, 24 months when stored at The invention further discloses preparation of gabapentin and isolation of gabapentin in polymorphic Form II with high yield and purity. A process for preparation of gabapentin comprising a step of obtaining 1,1-cyclohexane diacetic acid monoamide from 1,1-cyclohexane diacetic acid anhydride, wherein said reaction is characterized by the use of Assay preparation— Remove and weigh the contents of not fewer than 20 Capsules. Transfer an accurately weighed portion of the powder, equivalent to about 100 mg of gabapentin, to a suitable volumetric flask, and dissolve the contents in Diluent with sonication, if necessary, for about 60 seconds. a wet granulation method for preparing stable gabapentin tablets includes: forming a mixture by dry mixing of a first portion of a binder with the gabapentin, one or more excipients, or a Filter, and degas. MakemL, of gabapentin in the Assay preparation, based on the label adjustments if necessary (see System Suitability under Chromatog-claim; and r U and r S are the peak responses obtained from the Assay raphy 〈621〉). preparation and the Standard preparation, respectively. Standard preparation—Dissolve an accurately
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |