Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
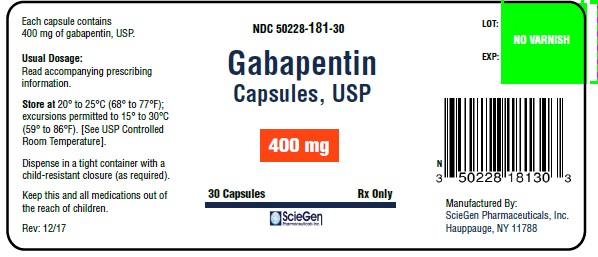
Gabapentin capsules, USP are supplied as follows: 100 mg: Capsules, with grey "100 mg" imprinted on the white body and grey "OE B56" on the white cap. The content is white or off-white powder. Gabapentin Capsules, USP is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. Gabapentin Capsules, USP is also indicated as adjunctive therapy in the treatment of partial seizures in pediatric patients age 3 to 12 years. Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). Gabapentin capsules, USP are a prescription medicine used to treat: Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Calculate the percentage of gabapentin related compound A in the portion of Capsules taken by the formula: Gabapentin Capsules, USP are available containing 100 mg, 300 mg or 400 mg of gabapentin, USP, supplied as follows: 100 mg capsules: Size '3' Hard gelatin capsules with white opaque cap and white opaque body, imprinted "100 mg" in blue ink on cap and "236" in blue ink on body, filled with white to off-white powder. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin USP. The inactive ingredients for the capsules are calcium carbonate, calcium sulfate dihydrate, glyceryl behenate, and pregelatinized starch. Store gabapentin capsules at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep gabapentin capsules and all medicines out of the reach of children. Gabapentin capsules are indicated for: • Management of postherpetic neuralgia in adults • Adjunctive therapy in the treatment of partial onset seizures, with and The active ingredient in gabapentin capsules, USP is gabapentin USP, which has the chemical name 1-(Amino methyl)-cyclohexane acetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin USP is a white to off-white powder. The active ingredient in Gabapentin Capsules USP is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin is a white to off-white crystalline solid with a pK a1 of 3.7 and a pK a2 Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. USP REFERENCE STANDARDS FOR PURCHASE USP Gabapentin RS Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Stopping gabapentin suddenly can cause serious problems. 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. How can I watch for early symptoms of suicidal thoughts and actions? USP Working standard solutions, Test solution, Chromatographic Gabapentin Related Compound A RS. system, and Procedure— Proceed as directed for Test 1 . Time: 30 minutes. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch, gelatin, magnesium stearate, mannitol, sodium lauryl sulphate, talc, titanium dioxide, black edible ink which contains iron oxide black, potassium hydroxide Gabapentin Capsules, USP are supplied as imprinted hard gelatin capsules containing 100 mg, 300 mg and 400 mg of gabapentin, USP. The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide. The 300 mg capsule contains FD&C Red 40, D&C Yellow 10 and titanium dioxide. Gabapentin capsules, USP, are indicated for: -management of postherpeticneuralgia in adults -Adjunctive therapy in the treatment of partial onset seizures, with and Gabapentin capsules, USP are for oral administration and contain 100 mg, 300 mg and 400 mg of gabapentin. In addition, each capsule contains the following inactive ingredients: croscarmellose sodium and magnesium stearate. Gabapentin Capsules, USP 300 mg are available for oral administration as hard gelatin capsules with a white opaque body and a yellow opaque cap. “APO 113” is imprinted on each capsule in black ink; supplied in:
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |