Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
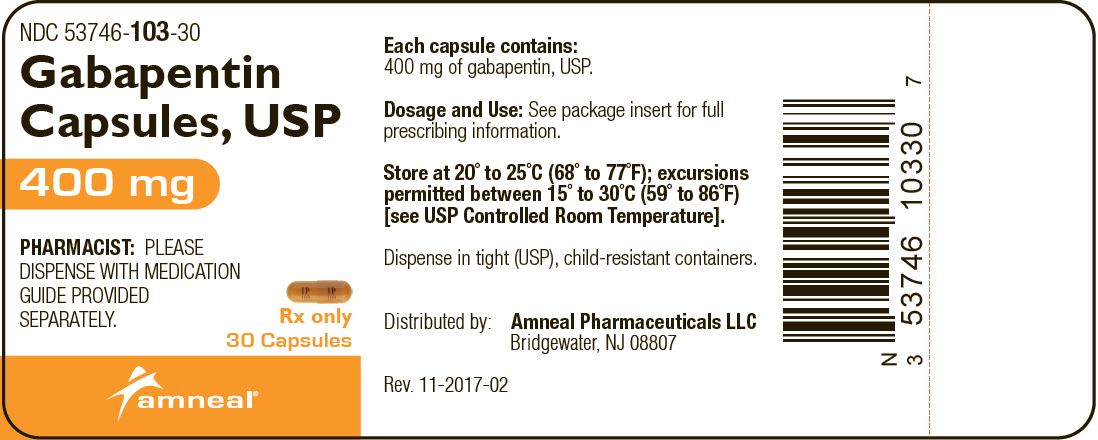
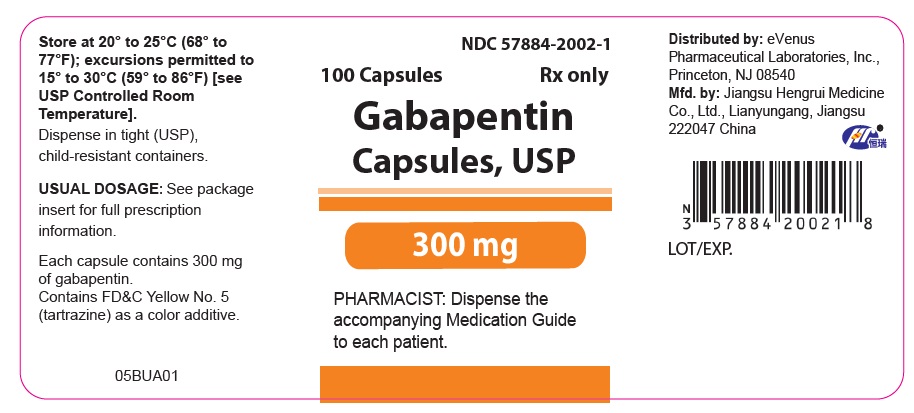
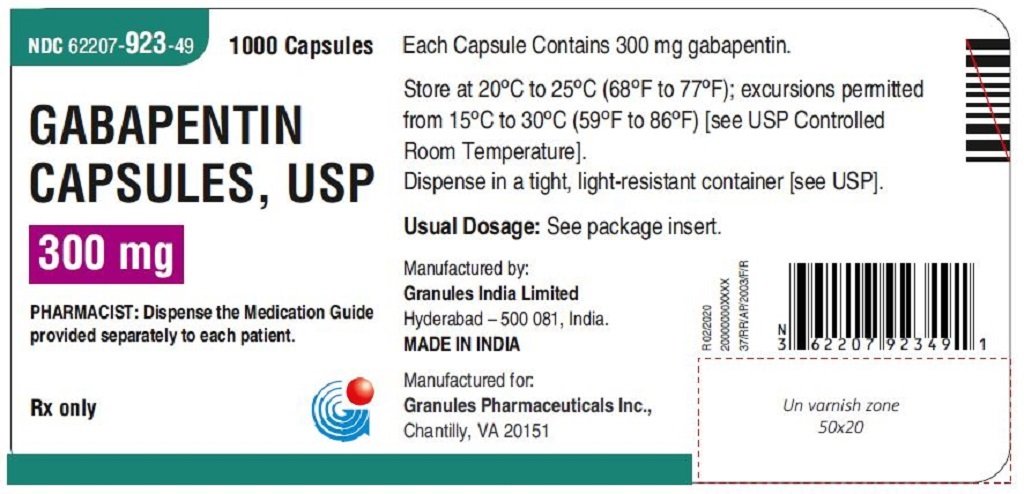
Gabapentin Capsules, USP DESCRIPTION Gabapentin Capsules, USP are supplied as imprinted hard gelatin capsules containing 100 mg, 300 mg and 400 mg of gabapentin, USP. The inactive ingredients are mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide. The 300 mg capsule contains FD&C Red 40, D&C Yellow 10 Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. Product Monograph: The Product Monograph is a scientific document that describes the properties, claims, indications and conditions of use of the product and contains any other information that may be required for optimal, safe and effective use. The Product Monograph includes three sections: Part I: Health Professional Information; Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. PRODUCT MONOGRAPH Prpms-GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg Gabapentin Tablets, USP 600 mg and 800 mg Antiepileptic Agent PHARMASCIENCE INC. 6111 Royalmount Ave., Suite 100 Montréal, Québec H4P 2T4 www.pharmascience.com Date of Revision: May 17, 2018 Submission Control No: 215700 PRODUCT MONOGRAPH PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Teva Standard Teva Canada Limited 30 Novopharm Court, Toronto, Ontario Date of Revision: December 6, 2017 Canada M1B 2K9 www.tevacanada.com Submission Control No: 211039 Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD APO-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Gabapentin Product Monograph Page 1 of 36 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGabapentin Capsules USP Capsules 100 mg, 300 mg, and 400 mg, oral The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 First Supplement which will be released 1 February 2010 and becomes official 1 August 2010. Should you have any questions, please contact Margareth Marques, Ph.D. (301-816-8106 or mrm@usp.org). Priva-GABAPENTIN Product Monograph Page 4 of 31 WARNINGS AND PRECAUTIONS General Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) is not considered effective in the treatment of absence seizures and should therefore be used with caution in patients who JAMP Gabapentin Capsules (Product Monograph) Page 1 of 28 PRODUCT MONOGRAPH PrJAMP Gabapentin Capsules Gabapentin Capsules Capsules, 100 mg, 300 mg, and 400 mg, Oral USP USP 35 Official Monographs / Gabapentin3297 Procedure—Separately inject equal volumes (about 20 µL) of. the Standard solution and the Test solution into the chromato-Gabapentin Capsules graph, record the chromatograms, and measure the responses for the major peaks. [NOTE—Disregard all the peaks having rela-» Gabapentin Capsules contain 2S (USP32) USP Gabapentin RS and USP Gabapentin Related Compound A RS Medium: 0.06N hydrochloric acid (prepared by adding 51mL in Diluent to obtain a solution having a known concentration of of hydrochloric acid to 10 L of water); 900mL. about 0.04mg of each per mL. Apparatus 2:50 rpm. Test solution—Weigh and finely powder not fewer than 20 Tab- Test specimen— Empty the contents of not fewer than 10 Capsules, and grind to a fine powder. Use an amount of the powder, equivalent to 2 mg of gabapentin, and 200 mg of potassium bromide. Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. the Standard solution and the Test solution into the chromato-Gabapentin Capsules graph, record the chromatograms, and measure the responses for the major peaks. pms-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT Mylan Pharmaceuticals ULC 85 Advance Road, Etobicoke, ON M8Z 2S6 Submission Control No.: 202563 Date of Revision: February 14, 2017 Gabapentin is used in combination with other anticonvulsants for management of partial seizures with or without secondary generalization in adults and children ≥3 years of age.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |