Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
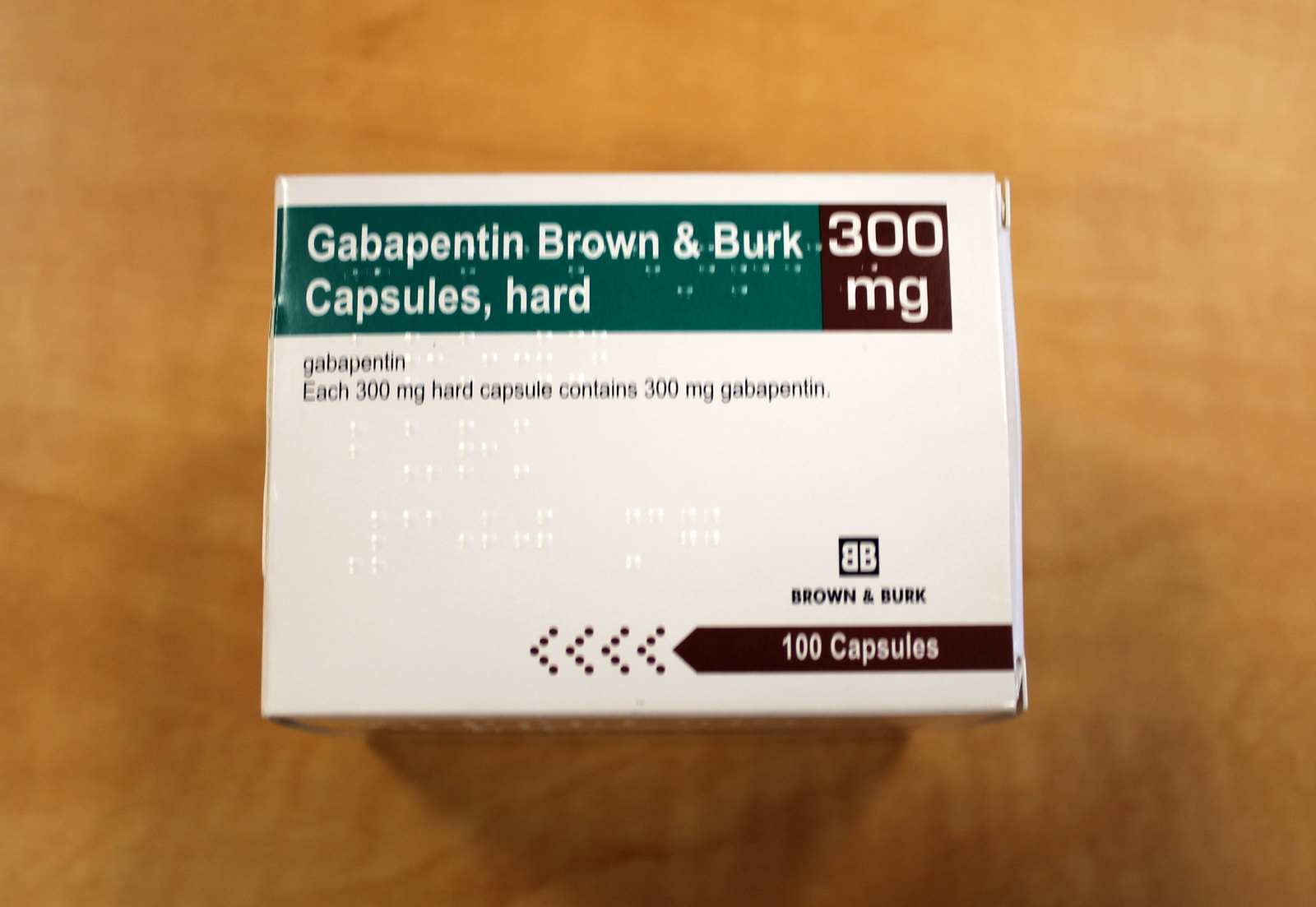 |  |
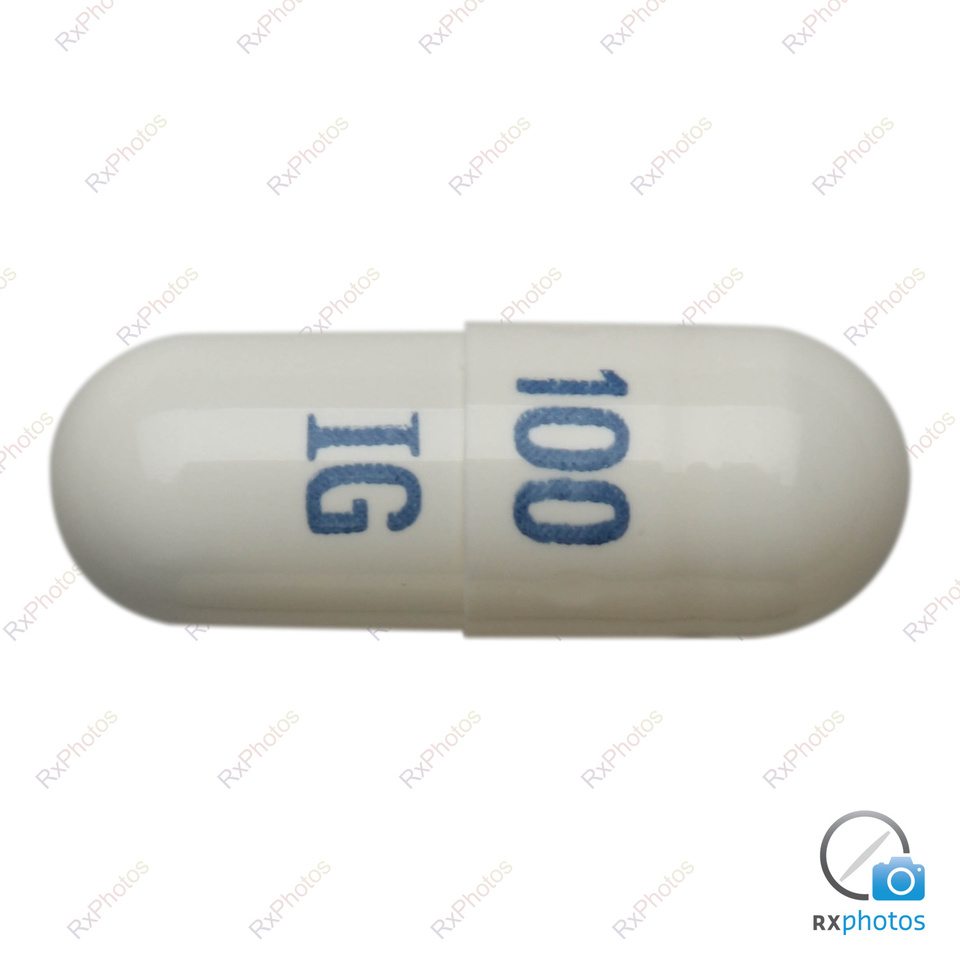
Co-administration of gabapentin with antacids containing aluminium and magnesium, reduces gabapentin bioavailability up to 24%. It is recommended that gabapentin be taken at the earliest two hours following antacid administration. Print SmPC information. (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. In a study involving healthy volunteers (N=12), when a 60 mg controlled- release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 Gabapentin is indicated as monotherapy in the treatment of partial seizures with and without secondary generalization in adults and adolescents aged 12 years and above. Treatment of peripheral neuropathic pain Gabapentin Capsule is indicated for treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults, as adjunctive therapy in treatment of partial onset seizures, with and without secondary generalization, in adults and pediatrics In a study involving healthy volunteers (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Dosage adjustment is recommended in patients with compromised renal function as described in Table 2 and/or those undergoing haemodialysis. Gabapentin 100 mg capsules can be used to follow dosing recommendations for patients with renal insufficiency. In a study involving healthy volunteers (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Gabapentine Sandoz capsule 100/300/400 mg, capsules, hard RVG 33677-33678-33679 V19 1.3.1.1 SmPC Juni 2024 Gabapentin treatment has been associated with dizziness and somnolence, which could increase the occurrence of accidental injury (fall). There have also been post-marketing reports of confusion, loss of consciousness and mental impairment. In a study involving healthy volunteers (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Gabapentin can be indicated meant for the treatment of peripheral neuropathic discomfort such since painful diabetic neuropathy and post-herpetic neuralgia in adults. Description of update: Type IB (C.I.2.a) variation application to update SmPC and PIL information in-line with the product information of reference product (Neurontin Hard capsule; EU reference No: DE/H/0899/001; MAH: Upjohn EESV) for Gabapentin Accord 100/300/400 mg hard capsules. PIL sections updated: 4, 6. No changes to SmPC. In a study involving healthy volunteers (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Dosage adjustment is recommended in patients with compromised renal function as described in Table 2 and/or those undergoing haemodialysis. Gabapentin 100 mg capsules can be used to follow dosing recommendations for patients with renal insufficiency. Print SmPC information. (N=12), when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule, mean gabapentin Gabapentin is indicated as monotherapy in the treatment of partial seizures with and without secondary generalization in adults and adolescents aged 12 years and above. Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin Capsules USP 300mg Technical Specification: Product Name: Gabapentin Capsules USP Brand Name: Generic Strength: 100mg, 300mg, 400mg Dosage Form: Capsules (Hard gelatin) Route of Administration: Via Oral Route Packing: 10s, 30s, 100s, 500s Pack Insert/Leaflet: PIL (Patient Information Leaflet), SmPC (Summ
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |