Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
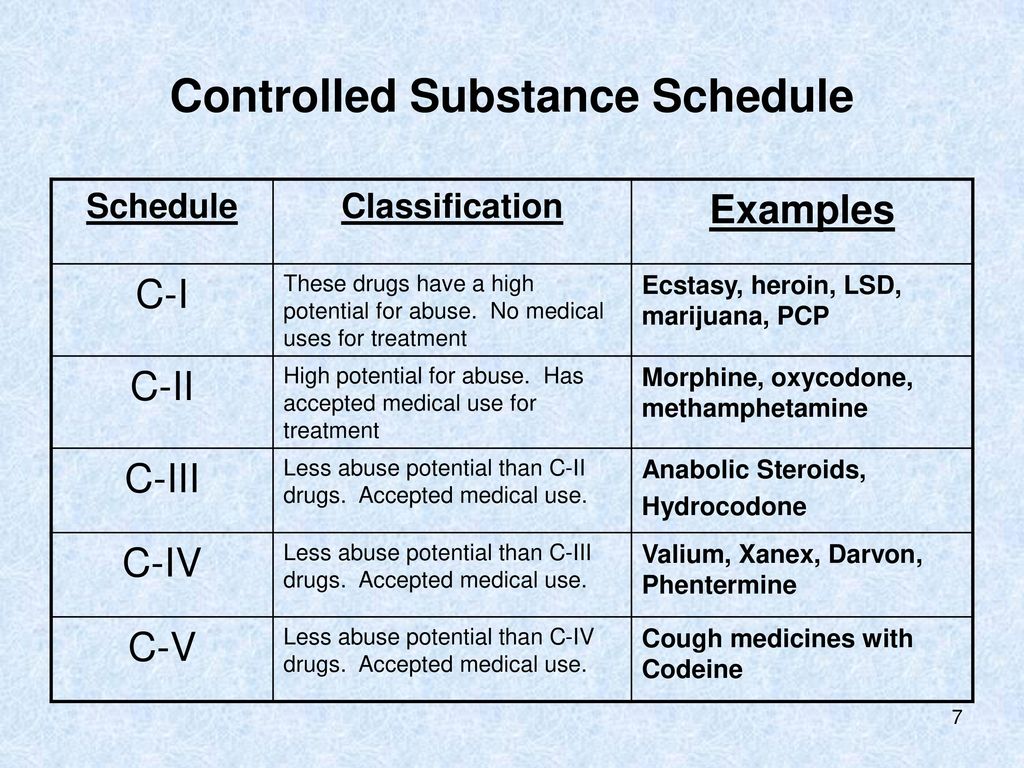 | 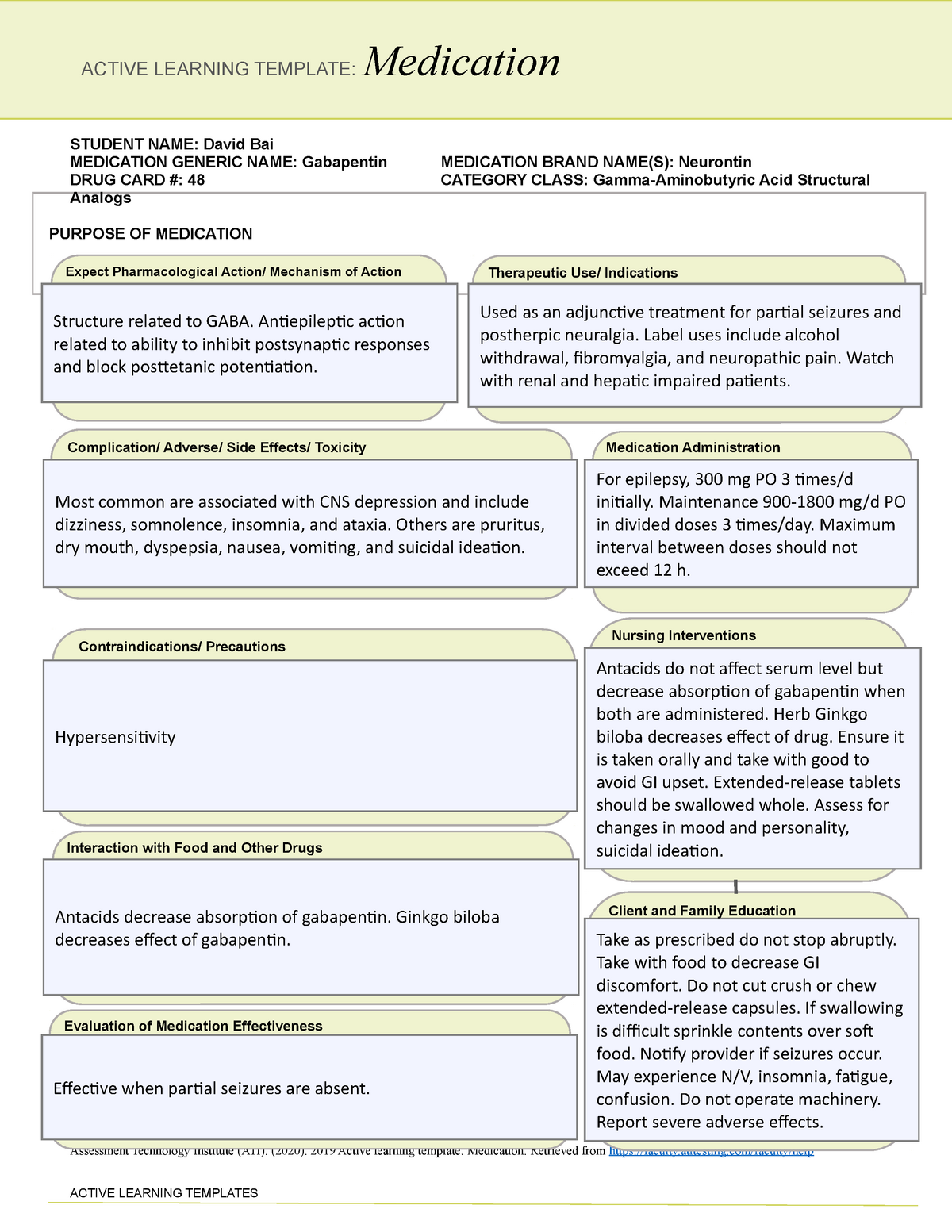 |
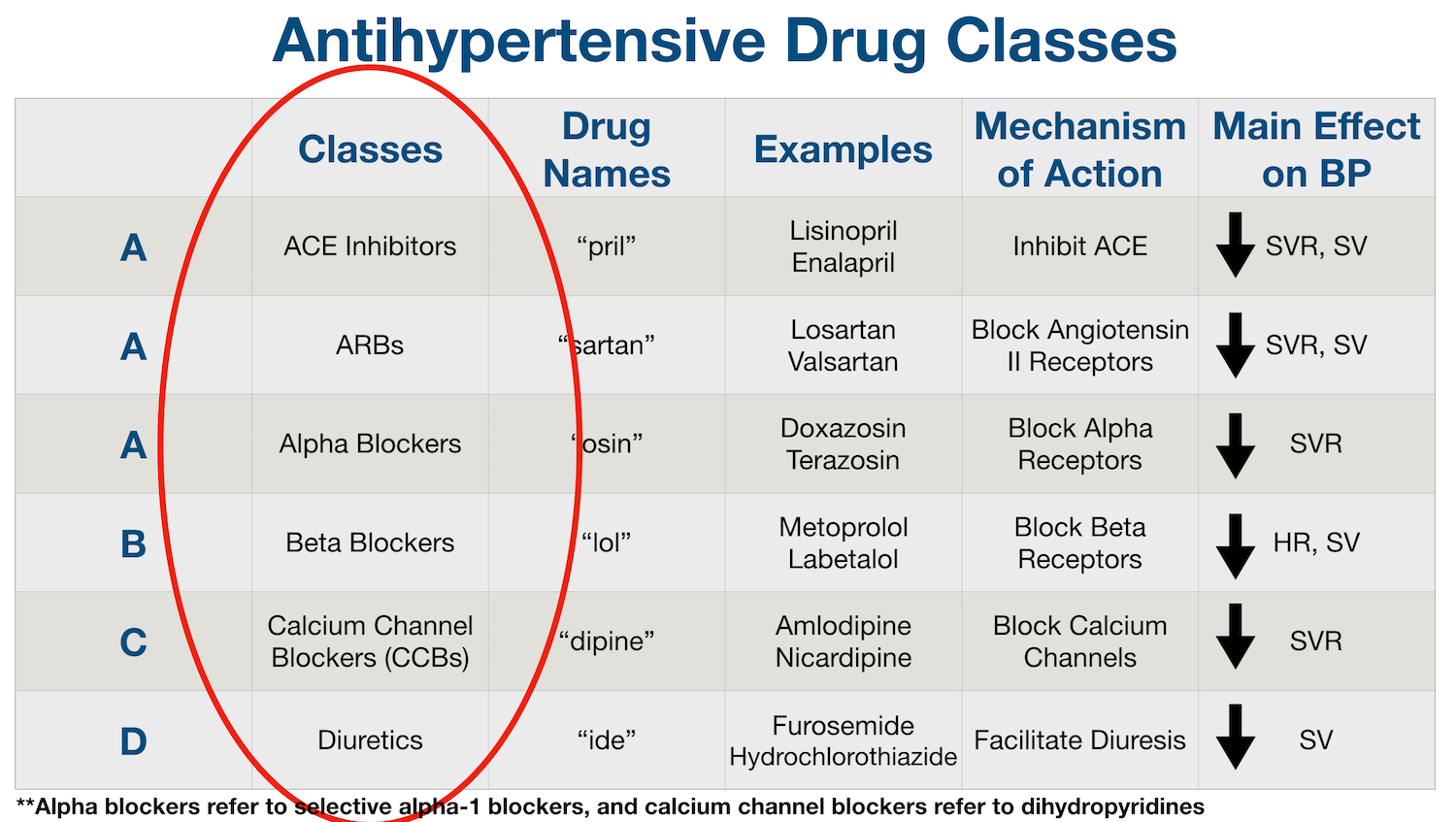
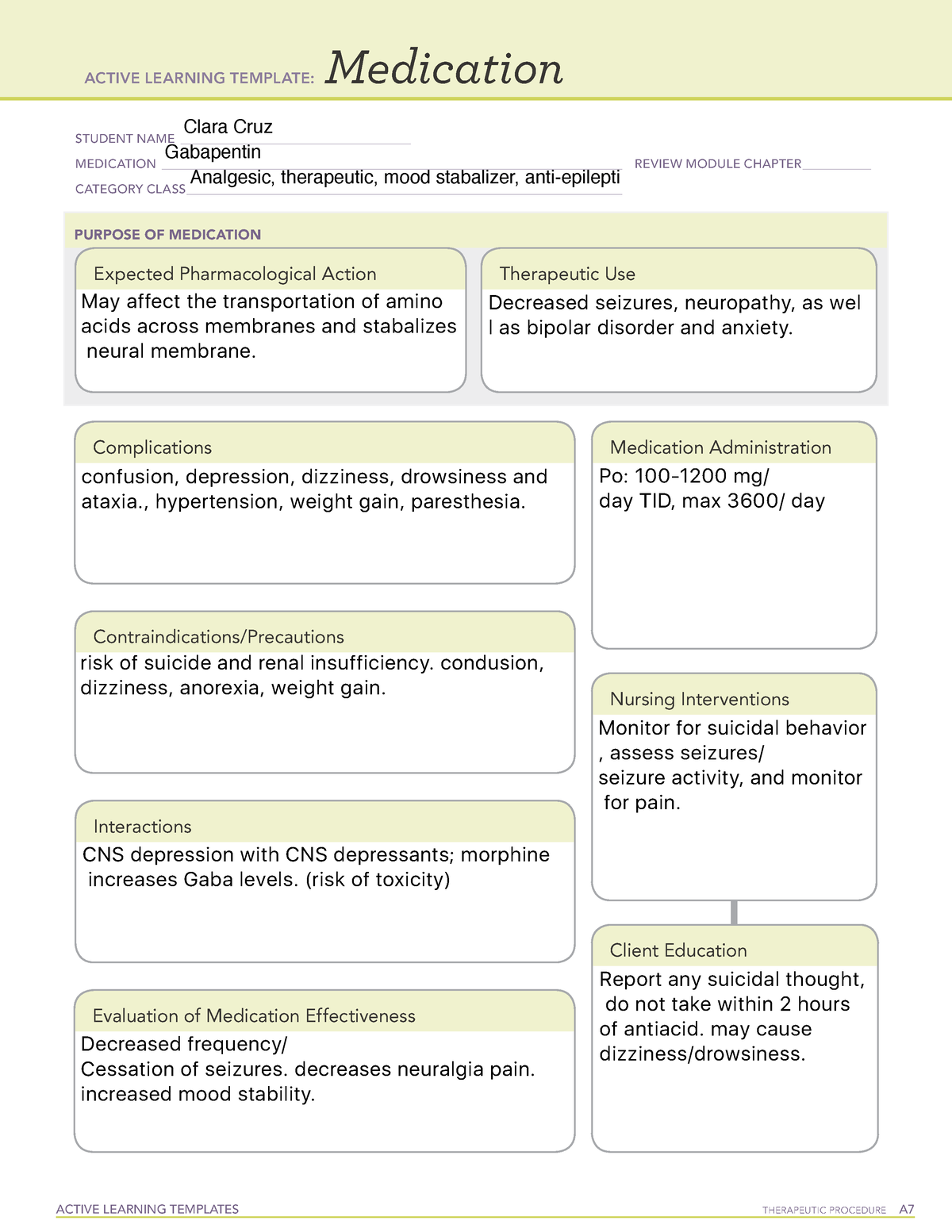
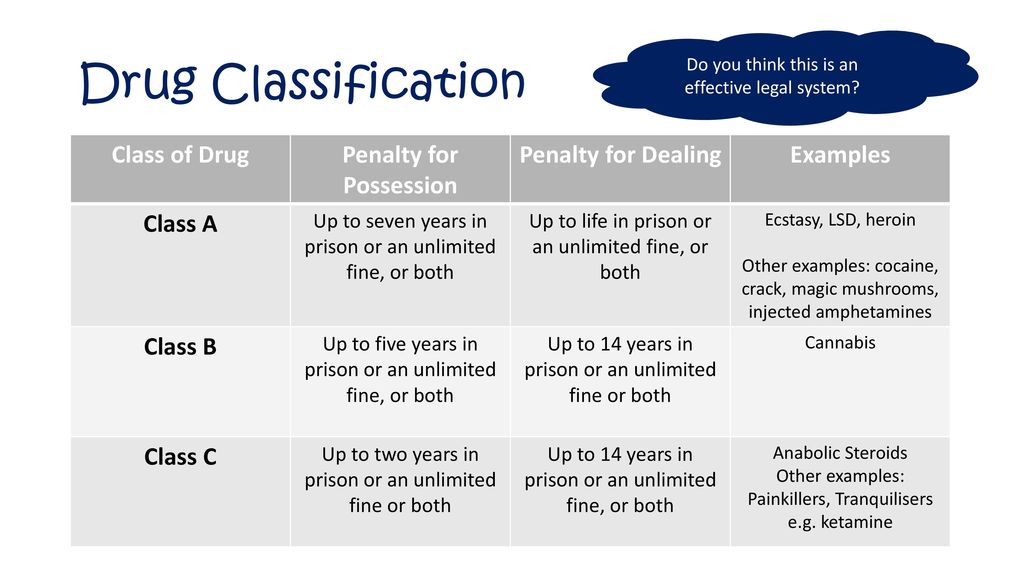
Pregabalin and gabapentin will be reclassified as class C controlled substances in the UK from next April to reduce the growing number of deaths associated with their misuse, the government has said.1 Victoria Atkins, parliamentary under secretary for crime, safeguarding, and vulnerability, said, “Any death related to the misuse of drugs is a tragedy. Pregabalin and gabapentin will be reclassified as class C drugs next year following a rising numbers of deaths linked to the drugs, the UK Government has said. The Home Office announced on 15 October that the prescription-only anti-convulsant medicines will be reclassified from April 2019. From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). Pregabalin and gabapentin will be reclassified as class C drugs next year following a rising numbers of deaths linked to the drugs, the Government has said. The Home Office announced yesterday (15 October) that the prescription-only anti-convulsant medicines will be reclassified from April 2019. From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after experts highlighted rising numbers of fatalities linked to the drugs. Last year, the government announced a change in the law that meant that gabapentin and pregabalin will be reclassified as Class C controlled drugs. The law change will come into force from April this year. What are Class C drugs? Class C drugs are a category of controlled drugs under the UK Misuse of Drugs Act 1971 that are seen to be the least harmful of substances to people, but still attract long prison sentences if found guilty in court, even for possession. Some of the most common Class C drugs include but are not limited to: As of 1 April 2019, pregabalin and gabapentin are classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 The list shows each drug’s respective classifications under both the Misuse of Drugs Act 1971 and the Misuse of Drugs Regulations 2001. The list is not exhaustive and, in the event of a Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations 2001 and Class C of the Misuse of Drugs Act 1971. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after experts Having originally been developed as an anti-seizure medication, gabapentinoids include gabapentin and pregabalin, which are now prescribed primarily for neuropathic pain, seizures and anxiety, but also for fibromyalgia, restless legs syndrome and complications of MS (Chan et al, 2023). The drugs – pregabalin and gabapentin – became illegal to possess without a prescription and illegal to supply or sell to others in the UK from April 2019. The move came after experts highlighted the potential for misuse, and rising numbers of fatalities linked to the drugs. However, concerns around the non-medical use (so-called “misuse”) of the gabapentinoids themselves are growing, as well as those pertaining to their iatrogenic dependence, diversion and toxicity. 7-17 In response, they were reclassified in the UK as Schedule II medications and Class C drugs in April 2019. 18, 19 Mixed methods qualitative The UK government is to reclassify the prescription drug pregabalin as a class C controlled substance, after experts issued safety warnings following an increase in deaths linked to its use. Patient Information Leaflet - Neurontin - (EMC website) GOV.UK - Pregabalin and gabapentin to be controlled as class C drugs; GOV.UK - Consultation outcome – pregabalin and gabapentin; The FDA (US) warns about serious breathing problems with gabapentin and pregabalin - US drug safety agency article from December 2019
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |