Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
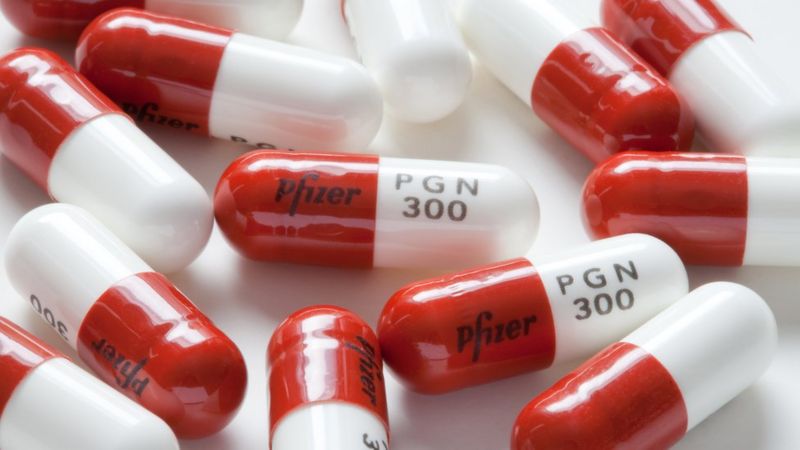
The overdose of gabapentinoids alone is less likely to cause respiratory depression but can be lethal in combination with other CNS depressants. 53 In response to concerns about medicinal misuse, diversion and addiction, pregabalin and gabapentin were reclassified as class C–controlled substances in the United Kingdom from 1 April 2019. 55 CLASS C (a) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a depressant effect on the central nervous system: (1) Chlordiazepoxide (2) Chlorhexadol (3) Clonazepam (4) Clorazepate (5) Diazepam (6) Flurazepam Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after Gabapentin is indicated for peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia, while pregabalin is indicated for both peripheral and central neuropathic pain. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. Neurontin (gabapentin) is used to treat pain you may have from shingles (postherpetic nerve pain). It is also used with other seizure medicines for partial onset seizures in patients 3 years and older. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy , postherpetic neuralgia , and central pain . [ 11 ] From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. pursuant to the texas controlled substances act, health and safety code, chapter 481, these schedules supercede previous schedules and contain the most current version of the As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. • The Home Office reclassified the prescription drugs pregabalin and gabapentin as class C drugs in April 2019 after concerns about misuse and addiction; • The change in law made it illegal to possess the drugs without a prescription, and supply or sell them to others; In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada, or Australia, and the other gabapentinoids, including phenibut, are not controlled substances Pregabalin and gabapentin are due to become controlled substances following a recent increase in the number of deaths attributed to the drugs. Last year, the Advisory Council on the Misuse of Drugs (ACMD) wrote to the government recommending the drugs to be reclassified as class C drugs because of the risks of “illegal diversion and medicinal Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin oral tablets and oral capsules should be stored at room temperature, between 68 F to 77 F (20 C to 25 C). It can be exposed to temperatures between 59 F to 86 F (15 C to 30 C), for The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |