Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
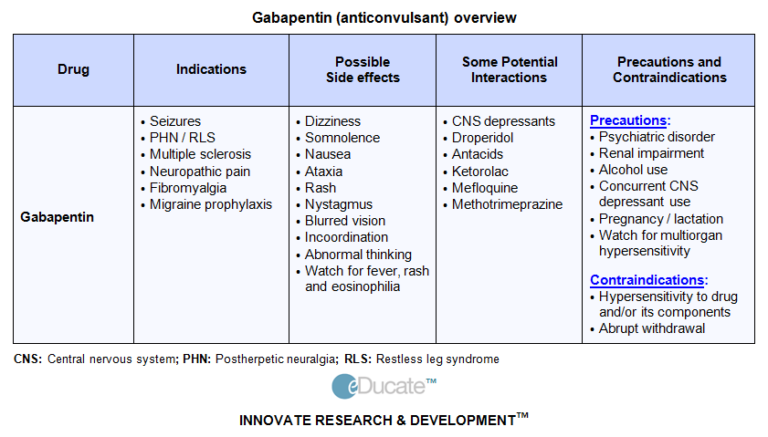
Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. This annual publication of the Texas Schedules of Controlled Substances was signed by David L. Lakey, Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be Published in the June 25, 2021 edition of the Texas Register (46 TexReg 3956) 2021 Schedules of Controlled Substances Decision Order Regarding the Modification of the Definition of Tetrahydrocannabinols in Schedule I of the Schedules of Controlled Substances The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- In this chapter: (1) "Board" means the Texas State Board of Pharmacy. (2) "Dangerous drug" means a device or a drug that is unsafe for self-medication and that is not included in Schedules I through V or Penalty Groups 1 through 4 of Chapter 481 (Texas Controlled Substances Act). (b) The director shall consult with the Department of State Health Services, the Texas State Board of Pharmacy, and the Texas Medical Board and by rule may: (1) remove a controlled substance listed in Schedules II through V from the official prescription program, if the director determines that the burden imposed by the program substantially Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin is an anticonvulsant medication prescribed for a variety of conditions. It is used to treat partial seizures‚ postherpetic neuralgia following shingles and restless legs syndrome. Gabapentin is available in both branded and generic forms. Gabapentin works by calming overactive nerves in your body. PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. Read this complete Texas Health and Safety Code - HEALTH & SAFETY § 481.104. Penalty Group 3 on Westlaw Federal and Texas law organizes controlled substances into five schedules or categories based on their potential for addiction and abuse. Drugs that are a greater threat are classified in Schedule I while the least dangerous drugs are put into Schedule V. Beginning March 1, 2020, pharmacists, and prescribers (other than a veterinarian) are required to check the patient’s PMP history before dispensing or prescribing opioids, benzodiazepines, barbiturates, or carisoprodol. These programs have been very effective in reducing abuse, misuse, and diversion of controlled drugs in Texas. The Texas Health and Human Services Psychiatric Drug Formulary is the publication that outlines the medications that have been approved for use in Community Mental Health Centers, Mental Health State Hospitals and the State Supported Living Centers. The formulary is updated and published at least annually. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. associated with gabapentin use could potentially decrease with statewide expansion of these programs. The use of therapeutic drug monitoring in routine clinical practice to optimize gabapentin’sefficacy and safety has not identified a relationship between gabapentin levels and Key Points Background: Gabapentin is an anticonvulsant used to Known hypersensitivity to gabapentin or its ingredients (4) -----WARNINGS AND PRECAUTIONS----- Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan ypersensitivity): Discontinue if alternative etiology is not established (5.1) •Anaphylaxis and Angioedema: Discontinue and evaluate patient Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug (b) The director shall consult with the Department of State Health Services, the Texas State Board of Pharmacy, and the Texas Medical Board and by rule may: (1) remove a controlled substance listed in Schedules II through V from the official prescription program, if the director determines that the burden imposed by the program substantially Texas HHS Psychiatric drug formulary. Antidepressants Antipsychotic gabapentin (Neurontin) guanfacine (Tenex, Intuniv) metoprolol (Lopressor, Toprol XL)
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |