Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
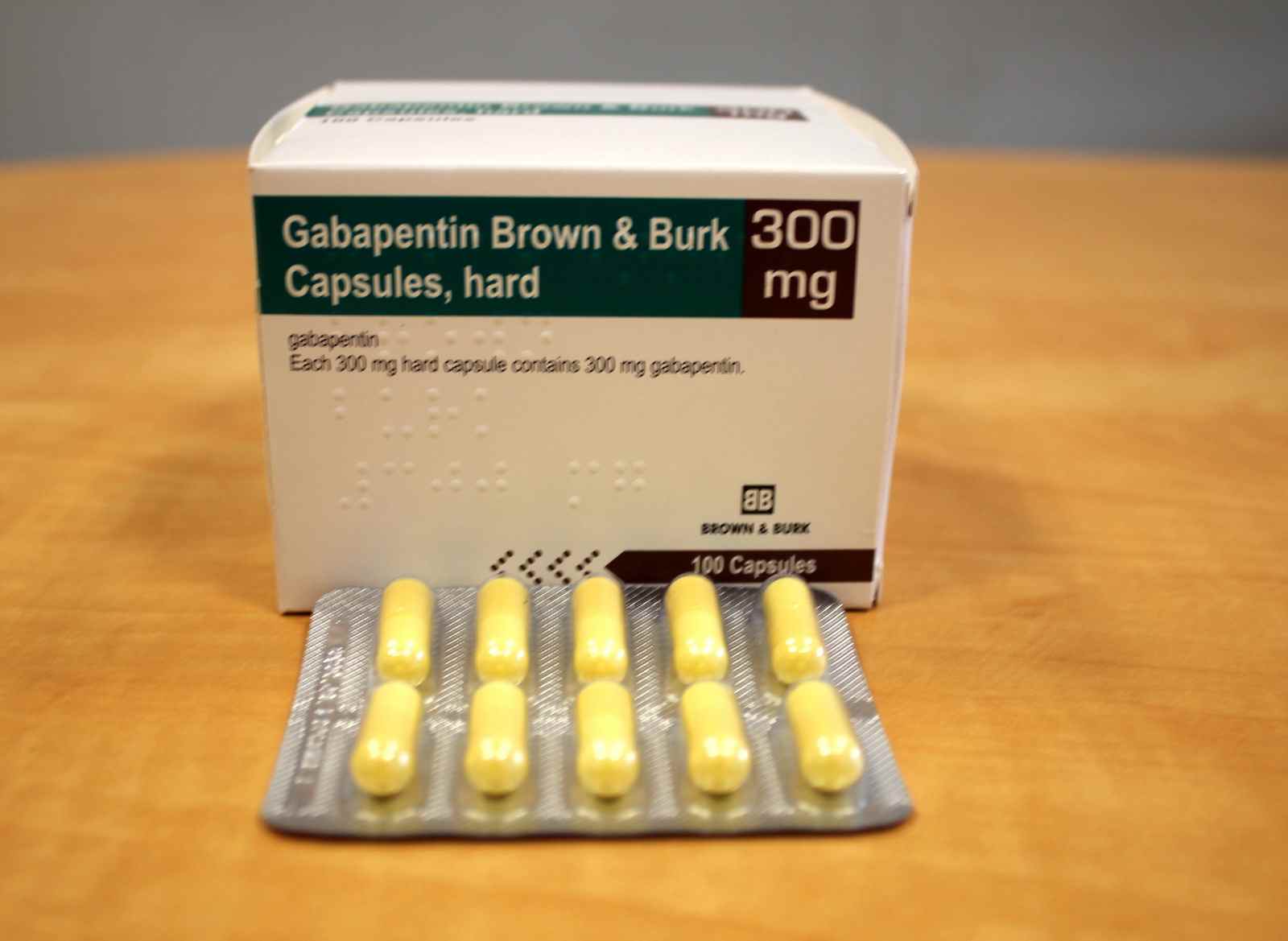
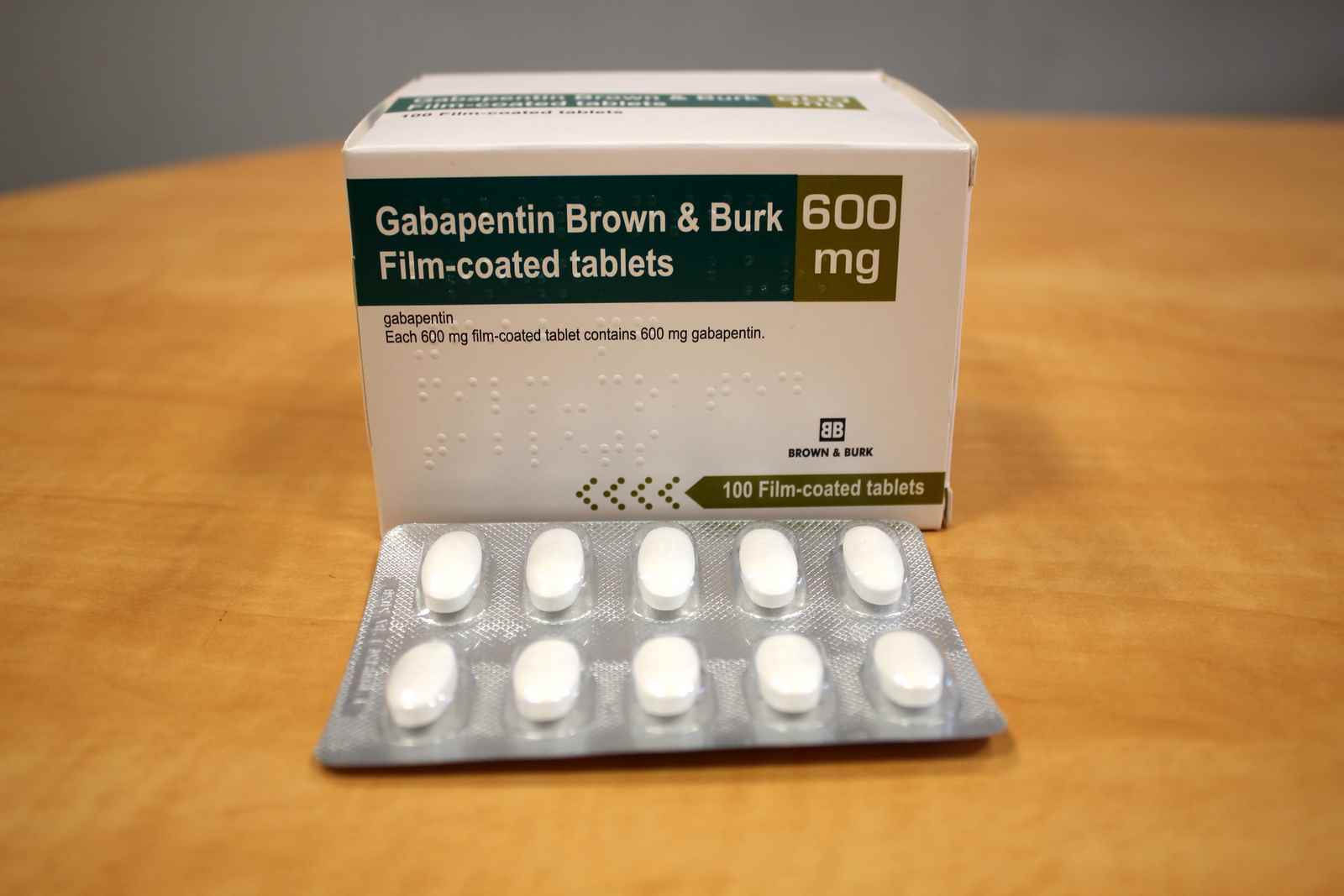
The government’s reclassification was expected to reduce prescribing of gabapentinoids, but Elisabeth Mahase finds that a lack of support for GPs and patients is limiting its impact In October 2018, the UK government announced that it would be reclassifying gabapentin and pregabalin1—known collectively as gabapentinoids—after experts pointed out the rising numbers of deaths linked to the Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how Courses Find your perfect course with these search options. The UK government reclassified gabapentin and pregabalin as ‘controlled drugs’ from April 2019. This study aimed to describe the trends in gabapentinoid prescribing before and immediately after reclassification, in the UK Clinical Practice Research Datalink, an electronic primary care health record broadly representative of the UK. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after MHRA/CHM advice: Gabapentin (Neurontin®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Neurontin (gabapentin) is used to treat pain you may have from shingles (postherpetic nerve pain). It is also used with other seizure medicines for partial onset seizures in patients 3 years and older. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. In January 2016, the ACMD recommended that gabapentin should be controlled under the Misuse of Drugs Act 1971 as Class C substances . Did you know? Like drinking and driving, driving while under the influence of drugs is illegal - with some drugs you can still be unfit to drive the day after using. As of 1 April 2019, pregabalin and gabapentin are classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 (as Gabapentin was first approved for use in the United Kingdom in 1993. [16] It has been available as a generic medication in the United States since 2004. [17] It is the first of several other drugs that are similar in structure and mechanism, called gabapentinoids. Gabapentin and pregabalin be placed under existing codes relating to “Other Class C” drugs as follows: Offences under s. 19 (a) and 19 (b) of the Criminal Justice (International Co-operation) Act GP clinical systems will be updated ahead of 1st April 2019, when pregabalin and gabapentin will be classified as CDs. NHS England has informed PSNC that EMIS has already implemented updates, meaning paper prescriptions will replace those previously sent by EPS. The list shows each drug’s respective classifications under both the Misuse of Drugs Act 1971 and the Misuse of Drugs Regulations 2001. The list is not exhaustive and, in the event of a ‘In April 2019, following a public consultation, as well as advice from the Advisory Council of the Misuse of Drugs, gabapentinoids were reclassified as Class C controlled substances and scheduled under the Misuse of Drugs Regulations 2001’. Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin The Home Office has placed pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019. Reclassification of pregabalin and gabapentin to Schedule 3 drugs from From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). The UK government is to reclassify the prescription drug pregabalin as a class C controlled substance, after experts issued safety warnings following an increase in deaths linked to its use. A Home Office consultation, which also proposes reclassifying gabapentin, has been launched in response to growing pressure for the drug to be reclassified to tackle misuse and addiction. Last year the
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |