Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 | 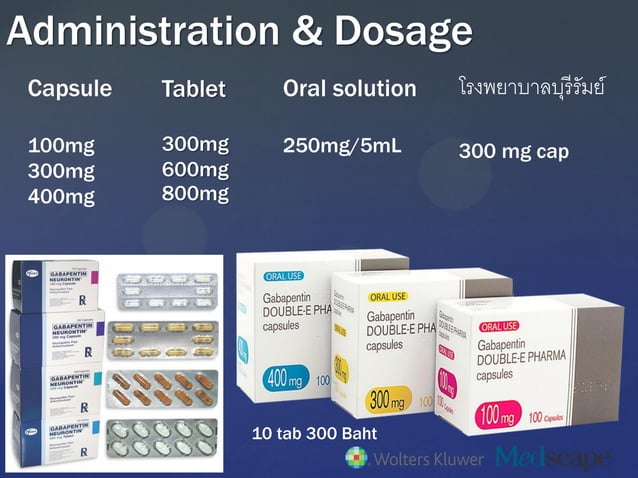 |
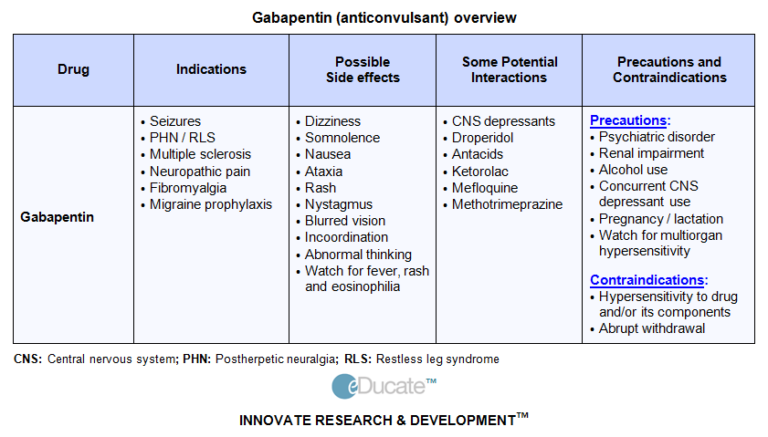
Abstract Background. Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided Selected References: Blotiere PO, et al. 2020. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 10(6). Brannon GE, Rolland PD. Anorgasmia in a patient with bipolar disorder type 1 treated with gabapentin. J Clin Psychopharmacol. 2000;20(3):379 In our study, only 28% of the women continued taking gabapentin throughout pregnancy as two-thirds of the women (66%) discontinued in the first trimester, most following pregnancy confirmation between 6 and 8 weeks’ gestation. IV Ketamine is contraindicated in the treatment of acute pain during pregnancy, despite its occasional use in anesthesia for labor and delivery. (Shwenk, 2018) IV lidocaine is in general considered safe, but there is limited data available on the safety of continuous lidocaine infusions in pregnant patients, as pregnancy was an exclusion From the limited amount of data in human pregnancy, it is not possible to inform an associated increased risk of congenital malformations because epilepsy itself and the presence of concomitant antiepileptic medicinal products have their own risks. Limited information indicates that maternal doses of gabapentin up to 2.1 grams daily produce relatively low levels in infant serum. Monitor the infant for drowsiness, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of anticonvulsant or psychotropic drugs. A single oral dose of either 300 mg or 600 mg given to NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 The combined evidence from this systematic review and animal studies raises concerns about the safety of using gabapentinoids during pregnancy. Careful evaluation of the benefit-risk balance for both mother and fetus/infant is essential when these medications cannot be avoided during pregnancy. There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. The American College of Obstetricians and Gynecologists (ACOG) has released guidelines on psychiatric medication used by women during pregnancy and lactation. The use of psychotropic medications Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. While gabapentin (Neurontin) is now used in a wide variety of clinical settings — for epilepsy, pain management, restless leg syndrome, anxiety, and sleep disturbance – there is relatively little information regarding its reproductive safety. Most recently, a prospective study from researchers at the Motherisk program reports on the outcomes of 223 pregnancies exposed to gabapentin Pregnancy: Based on animal data, may cause fetal harm. (8.1) NEURONTIN is contraindicated in patients who have demonstrated hypersensitivity to the drug To provide evidence on the safety of gabapentin use in pregnancy, we conducted a large cohort study of pregnant women within the US Medicaid Analytic eXtract (MAX) and assessed a range of neonatal and maternal outcomes. Gabapentin is not generally recommended in pregnancy as there is not enough information about whether it's safe for your baby. However, from the small amount of information that is available, there's no clear evidence that it's harmful. It should only be taken if the benefits of the medicine outweigh the risks.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |