Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 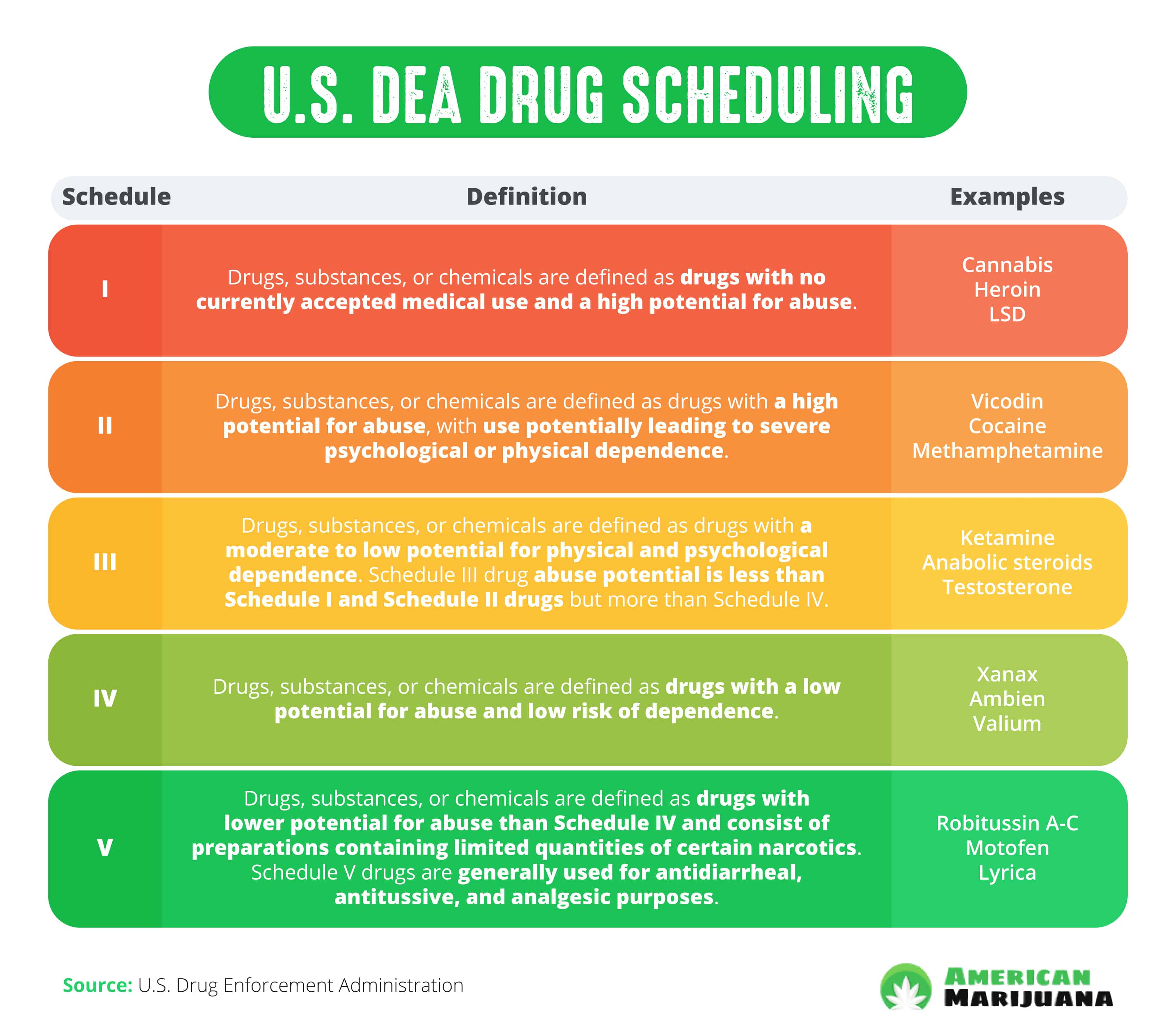 |
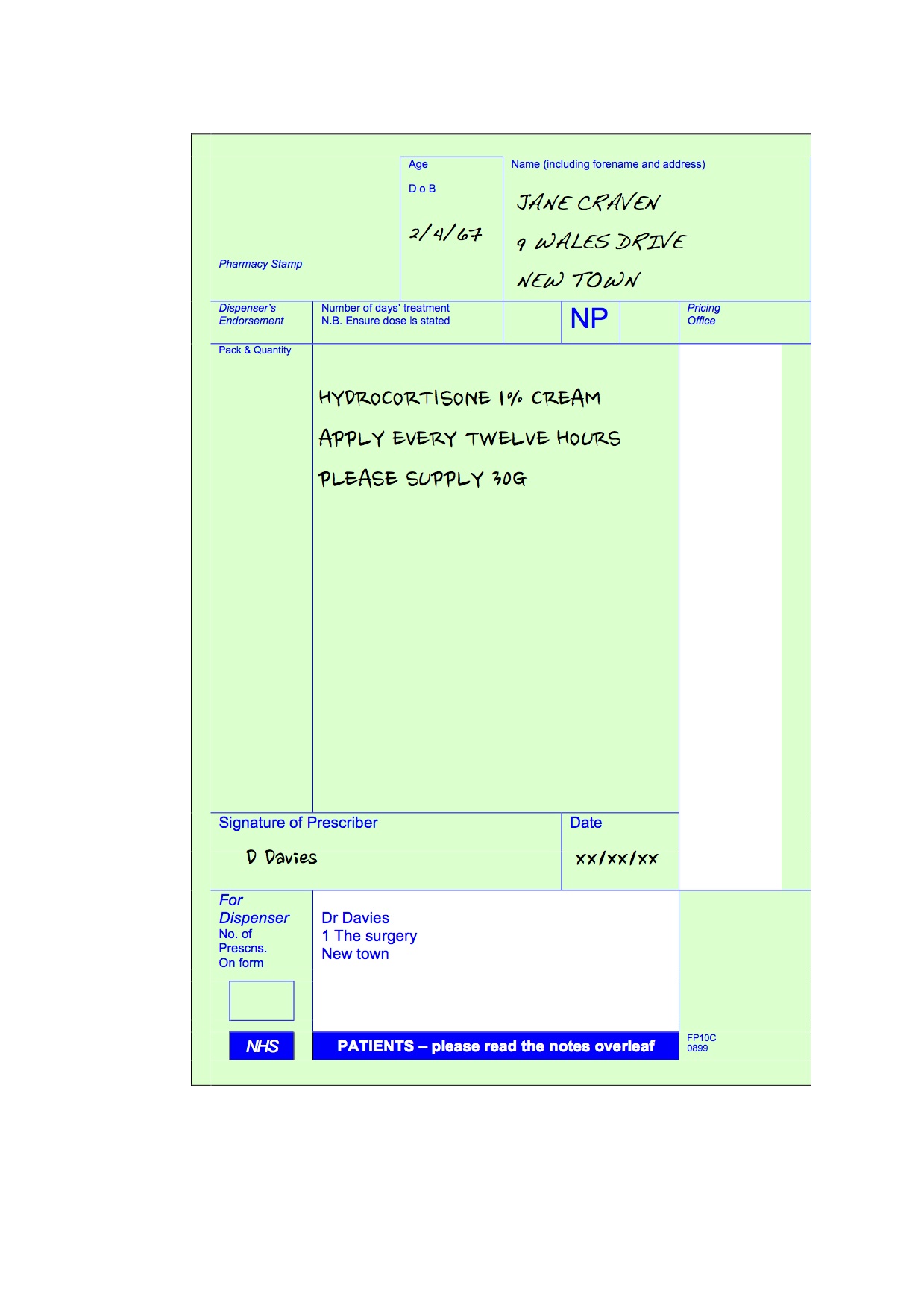
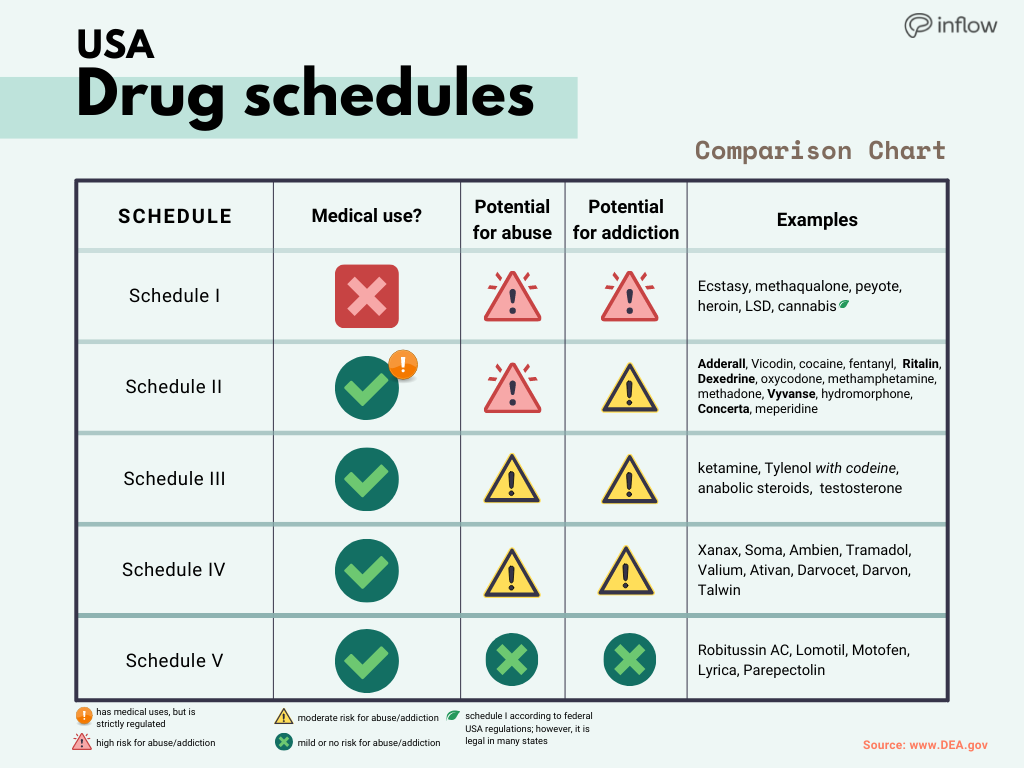
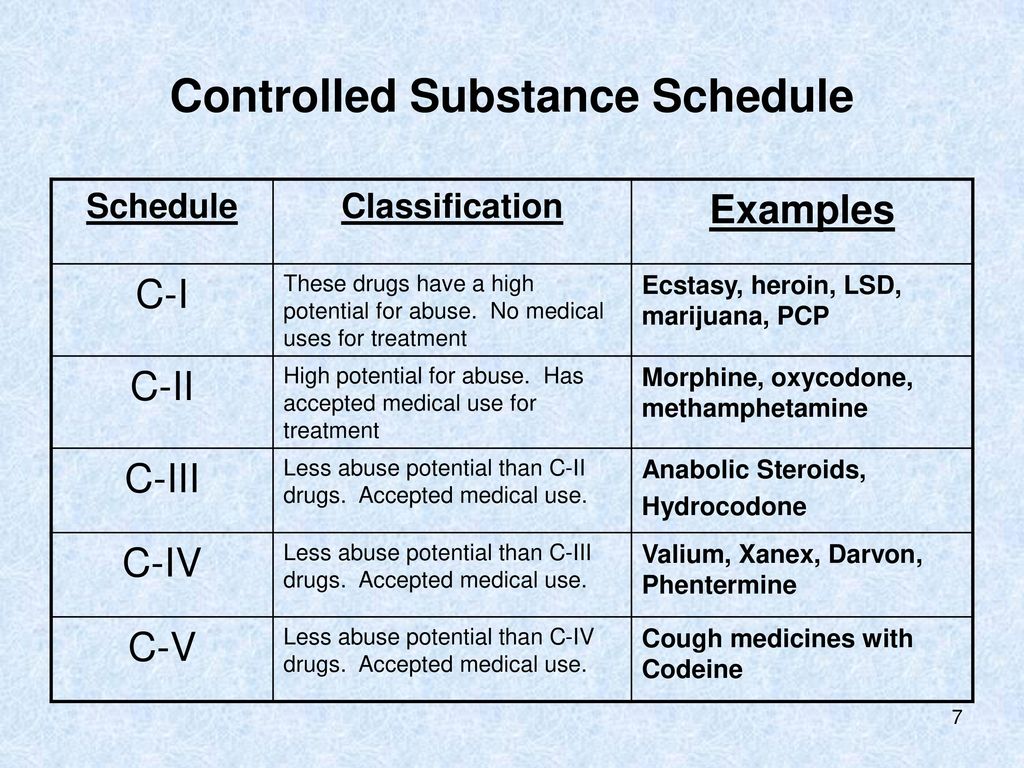
, any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and • Schedule V drugs have an even lower potential for abuse, but may include a limited quantity of narcotics. Examples include some cough medicines (Robitussin AC) and Lyrica. WHAT DRUGS DO PDMPs MONITOR? PDMPs track Schedule II, III and IV drugs in every state, and Schedule V drugs in 33 states and D.C., including Pennsylvania. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. nalbuphine (Schedule IV in KY, not scheduled federally) gabapentin (Scheduled V in KY, not scheduled federally) barbital, methylphenobarbital and phenobarbital (Schedule III in KY, Schedule IV federally)--Be advised--KY does not exempt ANY butalbital product from the Control Substance Act (902 KAR 55:045) services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Background In January 2016, the Advisory Committee for the Misuse of Drugs recommended that pregabalin and gabapentin are scheduled as Sch 3 CDs within the Misuse of Drugs SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Private prescriptions. Private prescriptions for Controlled Drugs in Schedules 2 and 3 must be written on specially designated forms which are provided by local NHS England area teams in England (form FP10PCD), local NHS Health Boards in Scotland (form PPCD) and Wales (form W10PCD); in addition, prescriptions must specify the prescriber’s identification number (or a NHS prescriber code in
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |