Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
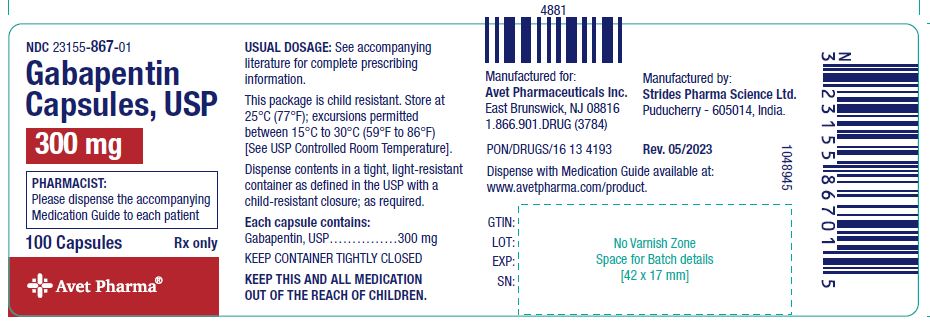
One of the most commonly prescribed drugs in human medicine, gabapentin also is used abundantly in veterinary practice. Starting Saturday, veterinarians in North Carolina are required to report when they dispense the drug gabapentin in quantities greater than a 48-hour supply. The Home Office has placed pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019. Pregabalin and gabapentin to be controlled as class C drugs. • Gabapentin dispensations for veterinarians can be reported using a state license failover. When reporting through PMP Clearinghouse leave the PRE01 and PRE02 fields blank and enter the veterinarian prescriber’s North Carolina registered veterinary license number in PRE04. The Environment Agency have advised that pharmacies and veterinary surgeons should apply for a T28 exemption certificate as it enables them to comply with the requirements of the Misuse of Drugs Regulations 2001 by denaturing controlled drugs prior to their disposal. The Drug Enforcement Administration (DEA) advises veterinarians to not provide their DEA license number for non-controlled substance prescriptions. Pharmacies that dispense from a veterinarian’s prescription should instead request the veterinarian’s license number. Q: I have received a vet prescription for gabapentin which is written on normal vet headed notepaper, is this legal? A: Yes it is acceptable. Veterinary prescriptions for a schedule 2 or 3 controlled drug do not have to be on a standardised FP10PCD form, however certain legal requirements must be present for it to be legally valid. Veterinary Telemedicine .0211 Read more NEWS: STOP Act Information per 90-113-74.(d) CONTROLLED SUBSTANCE REPORTING SYSTEM Dispensing of controlled substances require reporting to NC DHHS CSRS. You may contact the CSRS Utilization team with questions / concerns at CSRS.Utilization@dhhs.nc.gov. DHHS Radiation Protection Memo The use of gabapentin in veterinary medicine has increased dramatically in the last several years. Despite its popularity, there is a narrow indication of its use in veterinary patients. There is also growing evidence that gabapentin is being diverted for recreational drug use, sometimes with fatal consequences. For veterinarians that are prescribing more than a 72 hour supply of insulin drugs, glucagon drugs, diabetes devices, diabetic ketoacidosis devices, gabapentin, and naloxone are all prescriptions that do not require mandatory look-up. Requirements do not apply because all the listed medications, including gabapentin, are non-controlled substances. North Carolina Gov. Roy Cooper has signed into law the Opioid and Substance Use Action Plan, which will require veterinarians to report gabapentin use, although the medication is not a scheduled drug. The law goes into effect for pharmacies on March 1, 2024, and for veterinarians on March 1, 2025. Gabapentin is an anticonvulsant drug effective in humans to control neuropathic pain. In veterinary medicine, is extra-label used in combination with other treatments to control seizures when other drugs are not effective, when drugs are toxic, or for neuropathic pain treatment and anxiety. • The veterinary state license failover for Gabapentin dispensation reporting to the NC CSRS will launch on January 8th, 2025. 3. Do I have to report direct administrations of Gabapentin? • No, direct administrations of Gabapentin do not have to be reported to the NC CSRS. 4. To add to this, gabapentin is well known for its ability to potentiate the effects of opioids, making it a highly diverted drug. Although gabapentin remains a noncontrolled drug at the federal level, states are starting to implement their own requirements for increased gabapentin oversight compared with other noncontrolled medications. 3 V controlled substance as of July 1, 2019. Until then, gabapentin remains a drug of concern classified as a Schedule VI controlled substance. On July 1, 2019, veterinary establishments that possess gabapentin must take a complete and accurate inventory of this drug at the opening of business in accordance with . 3404 and §54.1- Gabapentin isn’t considered a controlled substance at a federal level. But several states have passed their own laws classifying gabapentin as a Schedule V substance. A written prescription for a POM-V that is not a controlled drug is valid for 6 months (unless a shorter period is stated). If the first dispense takes place during the written prescription validity period, the remaining repeats on the prescription may be dispensed beyond the validity period. This is also reflected in VMD Guidance. Controlled drugs It is acceptable to issue a further prescription for that controlled drug without a physical examination, however veterinary surgeons should carry out a further clinical assessment to ensure they have enough information to do so safely and effectively (see RCVS Controlled Drugs Guidance - A to Z for further guidance on controlled drugs). 4.22 Gabapentin is usually used to manage chronic pain, especially nerve-related pain. It is also used (primarily in cats) to relieve anxiety associated with veterinary procedures, travel, and other fear-generating situations. Gabapentin can also be used as an additional medication in seizure management. Before prescribing drugs like gabapentin and xylazine to your patients, get an overview of the basic regulatory structure and impact on these drugs. In veterinary medicine, gabapentin is utilized for both acute and chronic pain. However, its use in this manner is not backed by strong scientific evidence. There are no FDA labeled indications for gabapentin use in animals, but it is noted as having extra-label use in dogs for ancillary therapy of refractory seizures and as an adjunctive
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |