Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 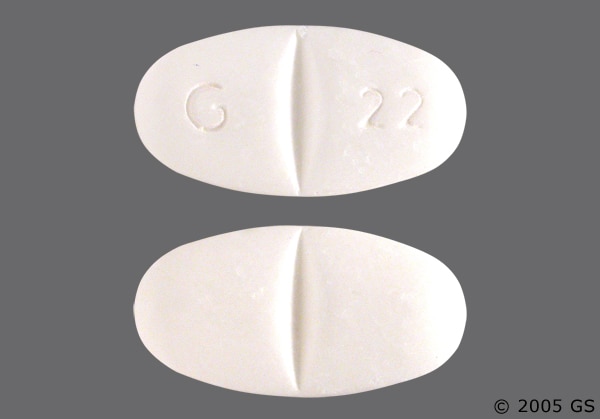 |
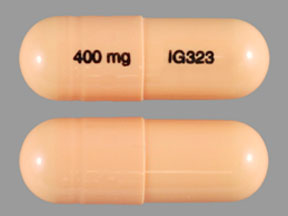
Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Gabapentin is used to control seizures in certain types of epilepsy and treat certain types of nerve pain. It is commonly prescribed to relieve nerve pain following shingles in adults, treating Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled substance because of its abuse potential as a non-opioid pain reliever . iii At least 26 States and the District of Columbia have Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid The prevalence of gabapentin abuse has been estimated to be 1.6% in the general population compared with 15% to 22% in patients with opioid use disorders. 23 Individuals with a history of substance abuse (particularly opioids) or mental illness have been identified as having an increased risk of abusing gabapentin. 23 Evidence of gabapentin abuse was observed in a group of 503 individuals in An alphabetical listing of controlled drugs by their brand, generic and/or street name that includes each drug’s schedule and the violation(s) from the Connecticut General Statutes (CGS) that are associated with each drug’s sale and/or possession. controlled substances, mandated by CT General Statutes Sec. 21a-254. Effective January 1, 2021, insulin drugs, glucagon drugs, diabetes devices, diabetic ketoacidosis devices, gabapentin, and naloxone are required to be uploaded into the CPMRS. Controlled substances are classified into 5 drug schedules which are determined by the U.S. Drug Controlled substances are classified as schedules I to V. Schedule I controlled substances have no recognized medical benefit, and are considered illegal drugs. Schedules II through V controlled substances, which include opioids and benzodiazepines, have a recognized medical benefit in the United States and some risk of addiction. report the dispensing of controlled substances and gabapentin, naloxone, and insulin drugs, glucagon drugs, diabetes devices, diabetic ketoacidosis devices. Note: Pursuant to Public Act 21-182, veterinarians that dispense will continue to report controlled substances, gabapentin, and other reportable drugs. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. For CSP registration information please read the DCP guide “Prescribing Controlled Substances in the State of Connecticut” at “Prescribing Controlled Substances in the State of Connecticut”. The prescription monitoring program cannot answer questions about the CSP registration or renewal process or prescriber status. Adding Gabapentin to the CPMRS will provide an additional data point to assist prescribers and pharmacists in the decision to prescribe or dispense a medication. At this time, the addition does not change Gabapentin to a controlled substance and therefore it would not be subjected to mandatory look-up prior to prescribing. The document below provides a compilation of the most commonly encountered controlled drugs, listed alphabetically by brand and generic and/or street name. This list contains the schedule in which each drug is classified, other names by which it is known, and the related criminal charge and penalty for possession and sale. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. This dispenser does not dispense; Schedule II-V controlled substances, insulin drugs, glucagon drugs, diabetes devices, diabetic ketoacidosis devices, gabapentin, or naloxone; or Schedule II-V controlled substances, insulin drugs, glucagon drugs, diabetes devices, diabetic ketoacidosis devices, gabapentin, or naloxone for greater than 48 hours. The Prescription Drug Monitoring Program’s mission is to collect and provide, by electronic means, access to controlled substance prescription data and other relevant data for the purpose of providing information to various stakeholders to promote the proper prescribing, dispensing and utilization of medications in Connecticut. Among the laws enforced by the Drug Control Division are the Pharmacy Practice Act, the State Food, Drug and Cosmetic Act, and the State Controlled Substances Act. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |