Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
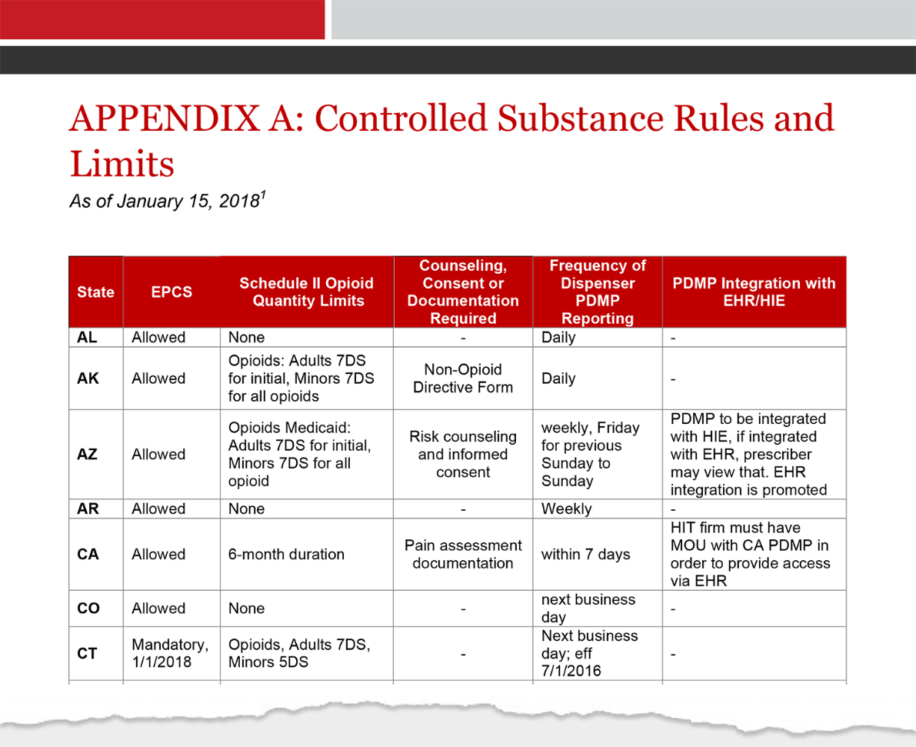 |  |
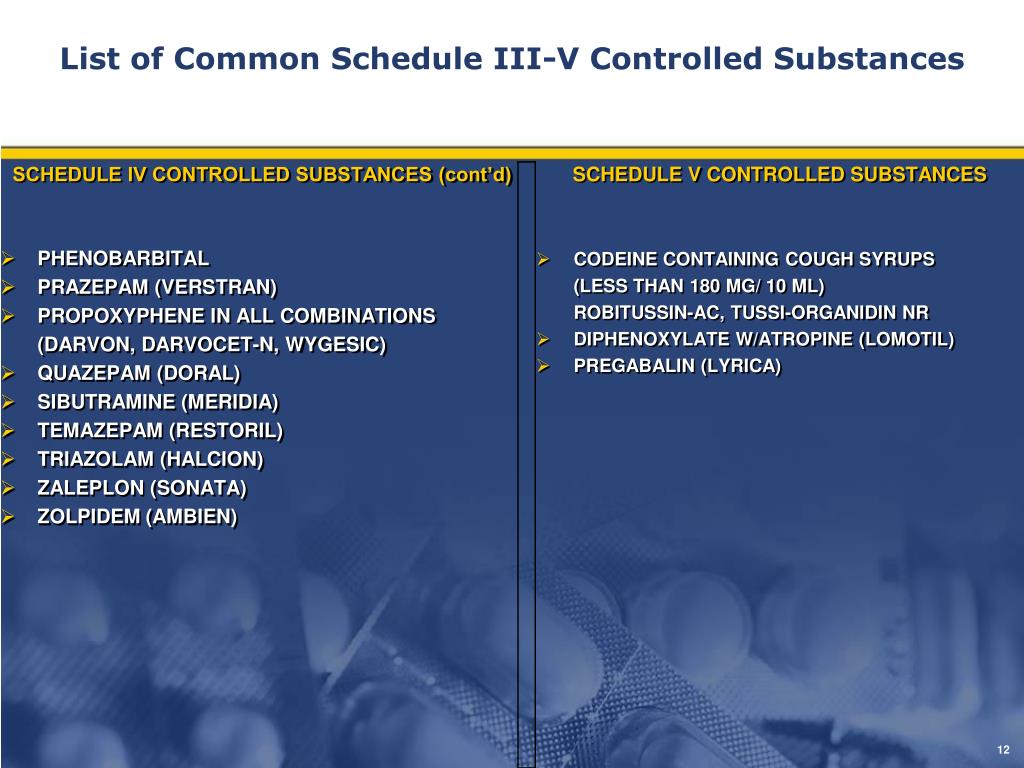
Established pursuant to N.J.S.A. 45:1-45 et. seq., the NJPMP is a statewide database that collects prescription data on Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH), and gabapentin dispensed in outpatient settings in New Jersey, and by out-of-State pharmacies dispensing into New Jersey. pharmacy for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin dispensed to an inpatient at a hospital, long-term care, or other facility in which the resident is provided with 24-hour nursing care. Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. The Division of Consumer Affairs (Division) is proposing to amend the Prescription Monitoring Program (PMP) rules to require New Jersey licensed pharmacies and registered out-of-State pharmacies to electronically transmit information to the Division about prescriptions filled for gabapentin. So, is Gabapentin a controlled substance? The state of Kentucky decided it would be in 2017. A controlled substance is when a drug is tightly managed by the government because it could lead to non-medical use and substance use disorders. Though, it’s classified as a Schedule V controlled substance, meaning it isn’t as addictive as other In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky Pursuant to N.J.S.A. 45:1-45 et. seq., and N.J.A.C. 13:45A-35.3, pharmacies that dispense Schedule II-V Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH), and gabapentin in New Jersey, or into New Jersey, are required to submit data on all transactions for such drugs to the New Jersey Prescription Monitoring Program (NJPMP). A pharmacy filling prescriptions in New Jersey in an outpatient setting for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin. i. For purposes of this subchapter, "human growth hormone" means somatrem, somatropin, or any analogue of either of them, consistent with 21 U.S.C. § 333 (e)4; 2. On May 7, 2018, the New Jersey Division of Consumer Affairs began requiring pharmacies begin reporting prescriptions for the unscheduled drug gabapentin to the New Jersey Prescription Monitoring Program (PMP). The drug, known under brand names Neurontin and Gralise, is currently unscheduled. The number of individuals who have Gabapentin present in an accidental overdose is significant enough . to add Gabapentin dispensation into the CPRMS. Note: The Department is not changing the controlled substance scheduling of Gabapentin at this time. As such, the CPMRS look-up requirements do not apply to Gabapentin prescribing. Naloxone The NJPMP, established pursuant to N.J.S.A. 45:1-45 et. seq., is a statewide database that collects prescription data on Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH) and gabapentin dispensed in outpatient settings in New Jersey, and by out-of-State pharmacies dispensing into New Jersey. Gabapentin is chemically known as -[1-(aminomethyl) 2 cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Overseen by the NJ Division of Consumer Affairs Statewide electronic database for collecting data from outpatient pharmacies on: Controlled Dangerous Substances (CDS) Schedules II, III, IV and V Human Growth Hormone Products Gabapentin (May 7, 2018) Operational since September 1, 2011 Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. If the person is a registrant, he/she shall list the controlled substance or substances which he/she desires to dispose of on DEA Form 41, and submit three copies of that form to the DEA Special Agent in Charge in his/her area; or registrants may contact the State's Drug Control Unit, receive instructions, complete a D.D.C. Form 51, and submit Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |