Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
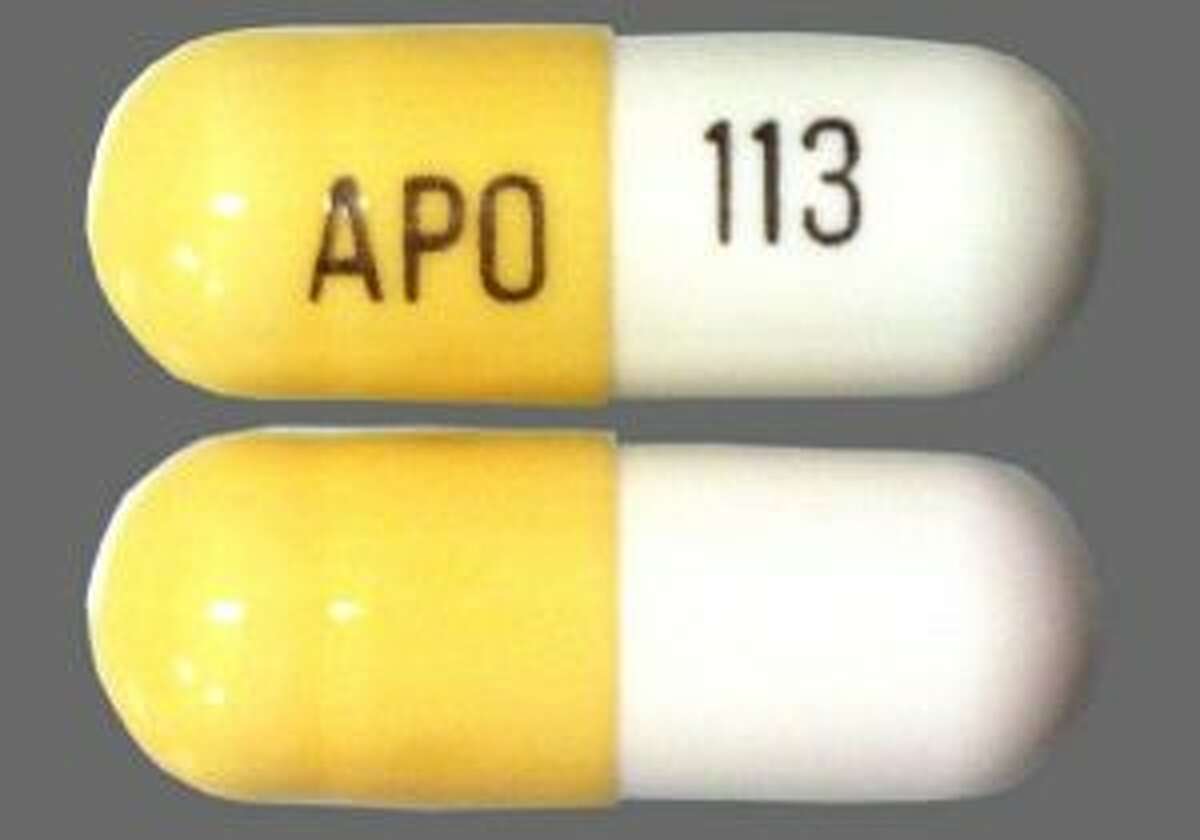
The Tennessee (TN) Controlled Substance Monitoring Database (CSMD) is a Prescription Drug Monitoring Program decreased 14%, note that gabapentin was added as a (d) Depressants, unless specifically exempted or excluded or unless listed in another schedule, means any material, compound, mixture, or preparation that contains any quantity of the following substances having a depressant effect on the central nervous system, including its salts: As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. substances in accordance with the requirements outlined by the DEA and state of Tennessee. Prescriptions for Gabapentin dated on or after July 1, 2018, should be tr e ad s controlled sub stance prescriptions and contain all c on tr lled ub ance requirements listed above. Remember to review all controlled substance record keeping, inventory, Justia › U.S. Law › U.S. Codes and Statutes › Tennessee Code › 2021 Tennessee Code › Title 39 - Criminal Offenses › Chapter 17 - Offenses Against Public Health, Safety and Welfare › Part 4 - Drugs › § 39-17-414. Controlled Substances in Schedule v In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky Justia Free Databases of U.S. Laws, Codes & Statutes. 2021 Tennessee Code Title 53 - Food, Drugs and Cosmetics Chapter 11 - Narcotic Drugs and Drug Control Part 3 - Regulations and Registration § 53-11-308. > Gabapentin Becomes Controlled Substance on July 1, 2018 > gabapentin_schedule_v175X100. June 21, 2018; The Tennessee Pharmacists Association (TPA) is the only Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. 0940-06-01-.01 controlled substances in schedule i. (1) Schedule I consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this rule. Justia Free Databases of U.S. Laws, Codes & Statutes. 2024 Tennessee Code Title 39 - CRIMINAL OFFENSES (§§ 39-1-101 — 39-17-1812) Chapter 17 - OFFENSES AGAINST PUBLIC HEALTH, SAFETY AND WELFARE (§§ 39-17-101 — 39-17-1812) Part 4 - DRUGS (§§ 39-17-401 — 39-17-456) Section 39-17-410 - Controlled substances in Schedule III additional controlled substances identified by the committee or commissioner (gabapentin, pregabalin, some stimulants) Tenn. Code Ann. § 53‐10‐310 (4) The controlled substances that trigger a check of the controlled substance database pursuant to subdivisions (e)(1) and (2) The Controlled Substance Monitoring Database (CSMD) is governed by the Tennessee Prescription Safety Act of 2016, Tenn. Code Ann. §§ 53-10-301 et seq., which authorizes the commissioner to promulgate rules as necessary for implementation of these statutes. Both have the force of law and may be used in the regulation of CSMD. On June 21, TPA released a member resource for pharmacists and pharmacy professionals, to assist practices regarding the scheduling of gabapentin as it becomes a schedule V controlled substance. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose Tennessee has taken specific steps to regulate gabapentin, affecting how it is prescribed, dispensed, and possessed. Understanding these rules is essential for both patients and healthcare providers to avoid legal complications. • Since 2013, the number of controlled substance prescriptions reported to the CSMD has decreased 15%, note that gabapentin was added as a new controlled substance in 2018 and had just over 2 million prescriptions reported in 2021. • Response time for searches in the CSMD was less than one second if the request did not include data
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |