Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 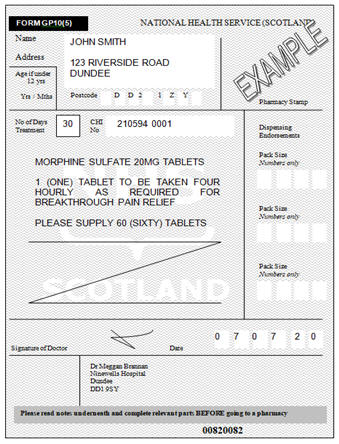 |
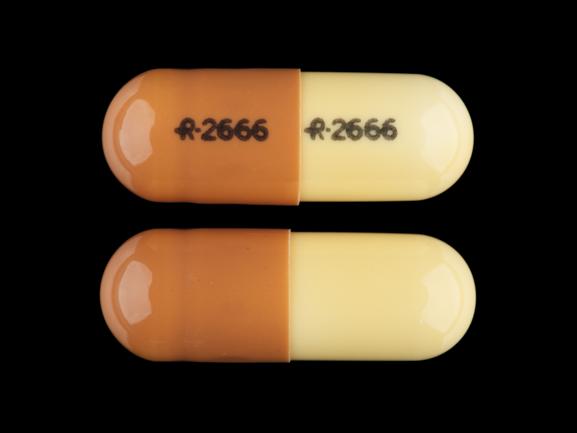
controlled drugs • Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from April 2019. What are Pregabalin and Gabapentin? They were originally used to treat epilepsy. They are also licenced to treat nerve pain and some types of anxiety. Why do we worry about it? From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. Summary Requirements for gabapentin and pregabalin from 1st April 2019 are as follows: Controlled Drug Prescription requirements Prescription validity From 1 April 2019, gabapentin and pregabalin have been reclassified as controlled drugs, leading to changes in how they are prescribed. Gabapentin and pregabalin are drugs used to treat a range of symptoms caused by MS, such as nerve pain, spasticity and spasms. Private prescriptions. Private prescriptions for Controlled Drugs in Schedules 2 and 3 must be written on specially designated forms which are provided by local NHS England area teams in England (form FP10PCD), local NHS Health Boards in Scotland (form PPCD) and Wales (form W10PCD); in addition, prescriptions must specify the prescriber’s identification number (or a NHS prescriber code in The UK government reclassified gabapentin and pregabalin as ‘controlled drugs’ from April 2019. This study aimed to describe the trends in gabapentinoid prescribing before and immediately after reclassification, in the UK Clinical Practice Research Datalink, an electronic primary care health record broadly representative of the UK. Updated to include additions to the list since December 2019. 2-β-Carbomethoxy-3-β- (4-Iodophenyl)tropane removed from list. Chloroamphetamine class changed from B to A. Epidyolex Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Are they controlled drugs? In short, yes they are controlled drugs but no, they do not need to be locked in a CD cabinet, recorded in a CD register or given with a witness. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Pregabalin and gabapentin will be reclassified as class C controlled substances in the UK from next April to reduce the growing number of deaths associated with their misuse, the government has said.1 Victoria Atkins, parliamentary under secretary for crime, safeguarding, and vulnerability, said, “Any death related to the misuse of drugs is a tragedy. Like gabapentin, it's taken for epilepsy and nerve pain. It can also be taken for anxiety. But there are differences between pregabalin and gabapentin. Pregabalin can be taken less often and in different doses to gabapentin. If you need to change to pregabalin, your doctor will explain how to swap safely from gabapentin. Following a consultation on the status of gabapentinoids, which closed in January 2018, the UK government has decided to reclassify the medicines but not to apply the requirements for safe custody. It is illegal to possess controlled substances without a prescription or to sell or otherwise supply them to others. The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. For more information about these As of 1 April 2019, pregabalin and gabapentin are classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 (as Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. The UK government is to reclassify the prescription drug pregabalin as a class C controlled substance, after experts issued safety warnings following an increase in deaths linked to its use. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after experts Gabapentin is chemically known as -[1-(aminomethyl) 2 cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. In October 2018, the United Kingdom (UK) reclassified pregabalin and gabapentin as class C controlled drugs, which will come into force in April 2019.1 This is in direct response to the increased number of deaths linked to both these drugs in the UK and a consultation process around this issue.2
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |