Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
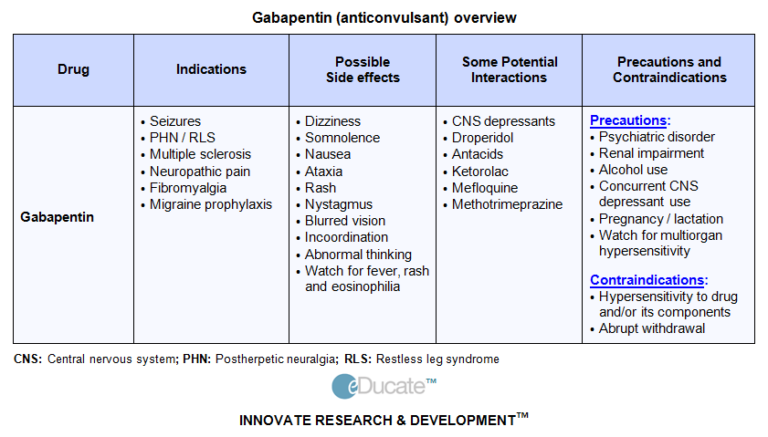 |  |
Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose By including gabapentin in the CSRS, prescribers can access all relevant information required to make the best clinical decision for their patients. 4. Do I need a DEA number to prescribe Gabapentin? • No, a DEA number is not required to prescribe Gabapentin. 5. Will Gabapentin prescription histories be available in the Controlled Substance Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§1308.11 through 1308.15. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada , or Australia , and the other gabapentinoids, including phenibut, are not controlled substances Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Controlled Substances - by Controlled Substance Code Number - SUBSTANCE CSCN CSA SCH NARC OTHER NAMES Codeine preparations - 200 mg/(100 ml or 100 gm) V Y Cosanyl,Robitussin A-C,Cheracol,Cerose,Pediacof Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Among cases classified as single substance pharmaceutical exposures (AAPCC defines single exposures as the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 7,214 cases in 2020. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The DEA’s drug schedule organizes drugs into groups based on risk of abuse or harm. Those drugs with high risk and no counterbalancing benefit are banned from medical practice and are Schedule I drugs. Conversely, those considered to have the lowest risk would be in Schedule V (5). Pharmacy Times interviewed Michael Abrams, MPH, PhD, senior health researcher at Public Citizen’s Health Research Group, to discuss his petition to the DEA and FDA regarding the classification of gabapentin and the closely related drugs, gabapentin enacarbil, as schedule V controlled substances. Estimates of steady-state pharmacokinetic parameters for phenobarbital or gabapentin (300 mg three times a day; N=12) are identical whether the drugs are administered alone or together. Naproxen Coadministration (N = 18) of naproxen sodium capsules (250 mg) with GABARONE (125 mg) appears to increase the amount of gabapentin absorbed by 12% to 15%. In 2019, the UK announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs. What other drugs will affect Neurontin? Using Neurontin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before using opioid medication, a sleeping pill, cold or allergy medicine, a muscle relaxer, or medicine for anxiety or seizures. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Any pharmacy, or licensed healthcare practitioner, who has a DEA number and dispenses gabapentin in (or into) Tennessee must report to the database daily (but no later than the close of business on the following business day) each controlled substance they have dispensed over the last 24 hours.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |