Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
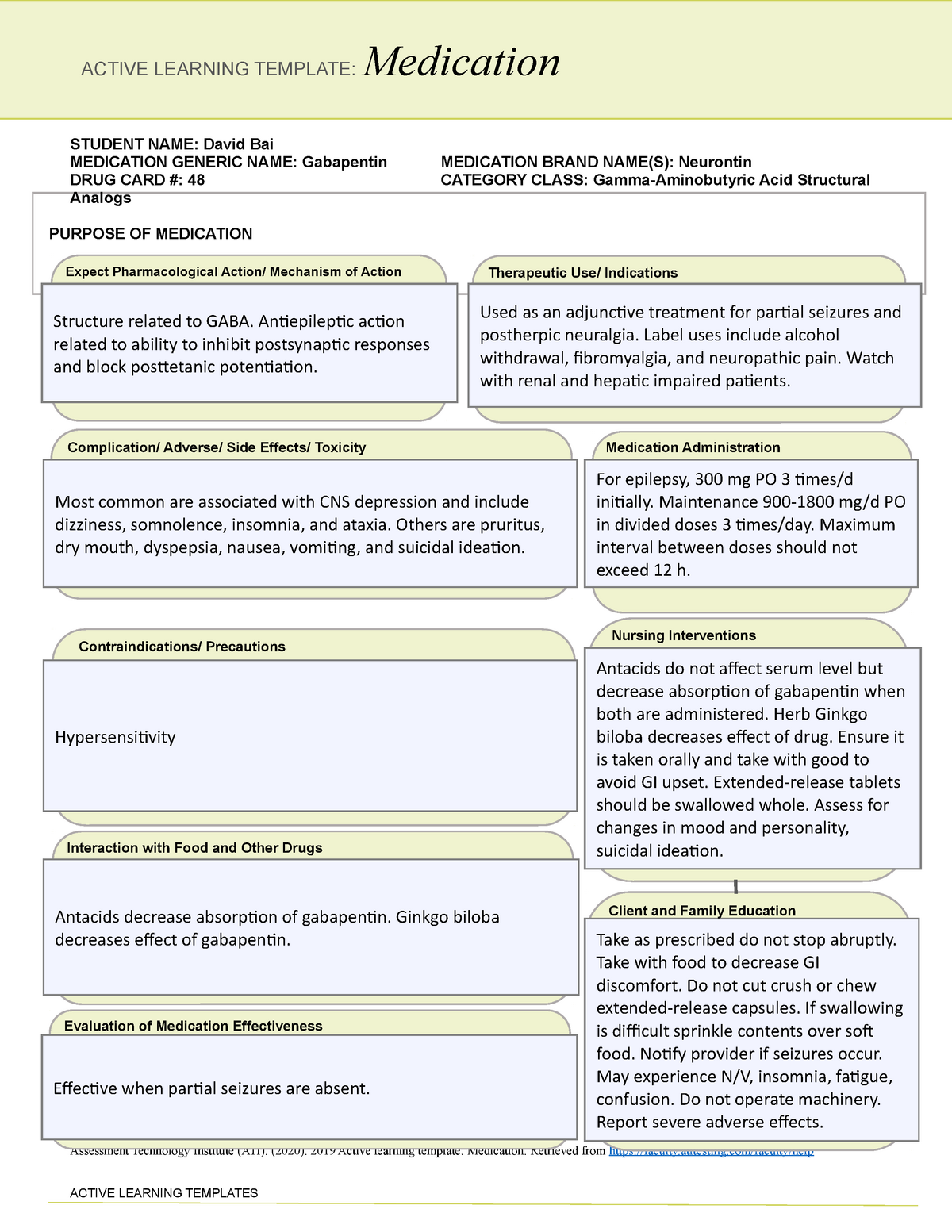
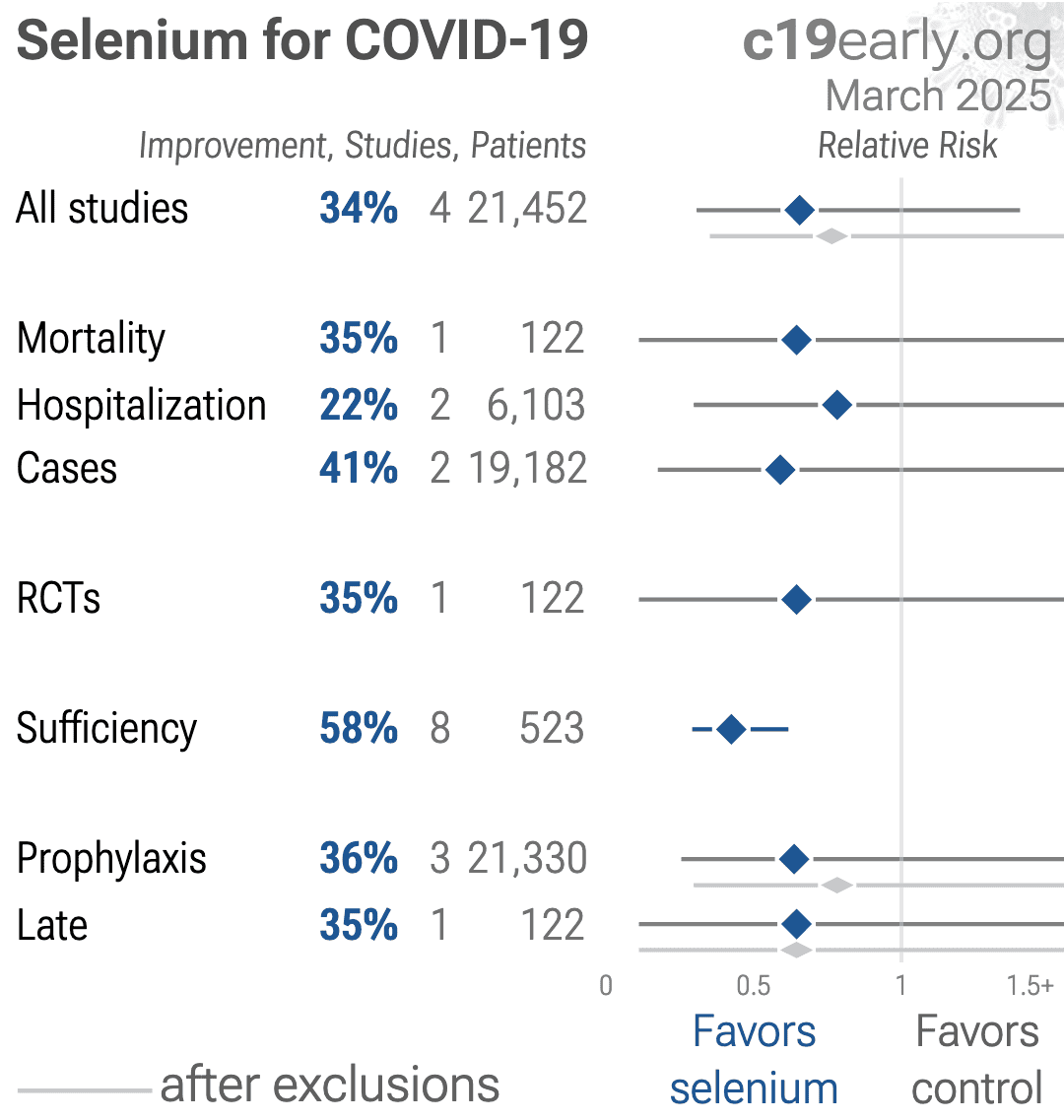
Patients exposed to gabapentin during pregnancy are encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334. Additional information is available at www.aedpregnancyregistry.org . Gabapentin is structurally related to the neurotransmitter GABA (gamma-aminobutyric acid) but it does not modify GABAA or GABAB radioligand binding, it is not converted metabolically into GABA Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. A lack of hepatic metabolism makes gabapentin an attractive option for patients on multiple antiepileptic drugs and patients with impaired hepatic function. Although gabapentin is relatively well tolerated, patients should be counseled on and monitored for CNS-related adverse effects, including somnolence, dizziness, ataxia, and asthenia. Gabapentin, like other gabapentinoid drugs, acts by decreasing activity of the α 2 δ-1 protein, coded by the CACNA2D1 gene, first known as an auxiliary subunit of voltage gated calcium channels. [13] [14] [15] However, see Pharmacodynamics, below. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Nevertheless, in renally compromised patients, the half-life may be considerably longer. 18 Since gabapentin is not bound to plasma proteins, there is no competition with other extremely protein-bound medications, for example phenytoin. 19 As well, the lack of hepatic metabolism or induction/inhibition of hepatic drug metabolizing enzyme Metabolism. Gabapentin is not appreciably metabolized. Gabapentin enacarbil, the prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Elimination Route. Excreted renally as unchanged drug. Half-life. Approximately 5–7 hours. Special Populations Gabapentin: Gabapentin is indicated for postherpetic neuralgia and serves as adjunctive therapy for managing partial seizures (with or without secondary generalization) in adults and pediatric patients aged 3 or older. High volume of distribution (58L), less than 3% of gabapentin is bound to plasma proteins : Metabolism: Not metabolized to a significant extent in humans. Elimination: Solely renal excretion as unchanged drug, and can be removed from plasma by hemodialysis. Half-life Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Gabapentin may increase the excretion rate of Mercaptopurine which could result in a lower serum level and potentially a reduction in efficacy. Mesoridazine: The risk or severity of CNS depression can be increased when Mesoridazine is combined with Gabapentin. Mestranol: The metabolism of Mestranol can be increased when combined with Gabapentin. Neither drug is metabolized by nor inhibits hepatic enzymes that are responsible for the metabolism of other drugs. Both drugs are excreted renally, with elimination half-lives of approximately 6 hours. Pregabalin and gabapentin both show dose-response relationships in the treatment of postherpetic neuralgia and partial seizures. The gabapentinoid drugs gabapentin and pregabalin are antiepileptic hypersensitivity and suppresses medial prefrontal cortical glucose metabolism . in rats with neuropathic pain. Mol Pharmacokinetics and Drug Metabolism All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans. Oral Bioavailability: Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Gabapentin is not a scheduled drug. Abuse. Gabapentin does not exhibit affinity for benzodiazepine, opiate (mu, delta or kappa), or cannabinoid 1 receptor sites. A small number of postmarketing cases report gabapentin misuse and abuse. These individuals were taking higher than recommended doses of gabapentin for unapproved uses. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. Gabapentin has some stark advantages as compared with other anti-epileptics, such as a relatively benign adverse effect profile, wide therapeutic index, and lack of appreciable metabolism making it unlikely to participate in pharmacokinetic drug interactions.. It is structurally and functionally related to another GABA derivative, . In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Only at the highest concentration Gabapentin's unique pharmacokinetic profile, characterized by its lack of hepatic metabolism and renal excretion, makes it a valuable antiepileptic drug with minimal drug-drug interactions. Its dose-dependent absorption and influence on neurotransmitter metabolism further contribute to its therapeutic effects.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |