Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/3L6TKOMCWZF7PDRJD6UMPCYE2A.jpg) |
/cloudfront-us-east-1.images.arcpublishing.com/gray/NOI7MVPV3BAXNCFGKUR6SBWXFU.jpg) | 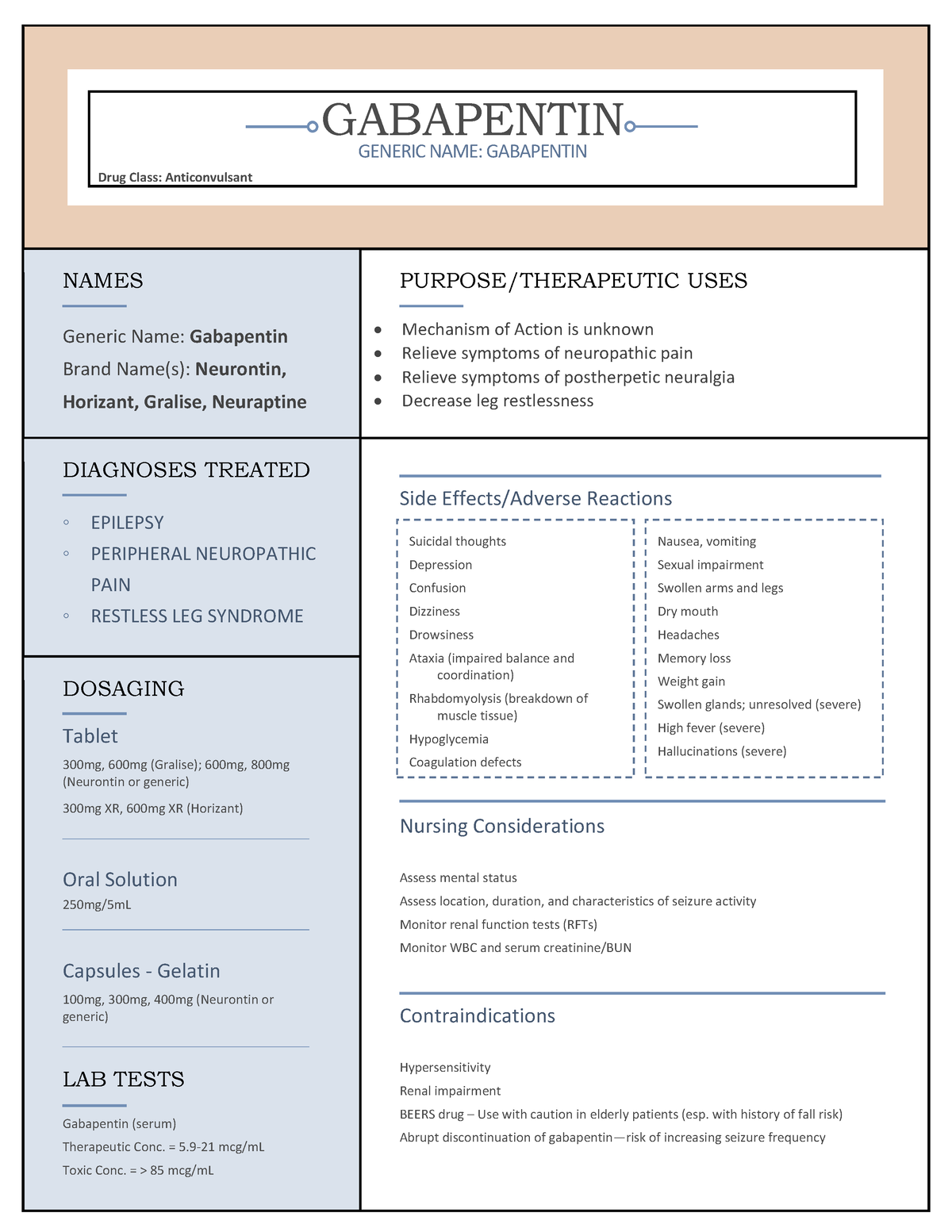 |
/cloudfront-us-east-1.images.arcpublishing.com/gray/VHHU3WK2VVHU3PWL43SCADA7TE.bmp) |  |
 |  |
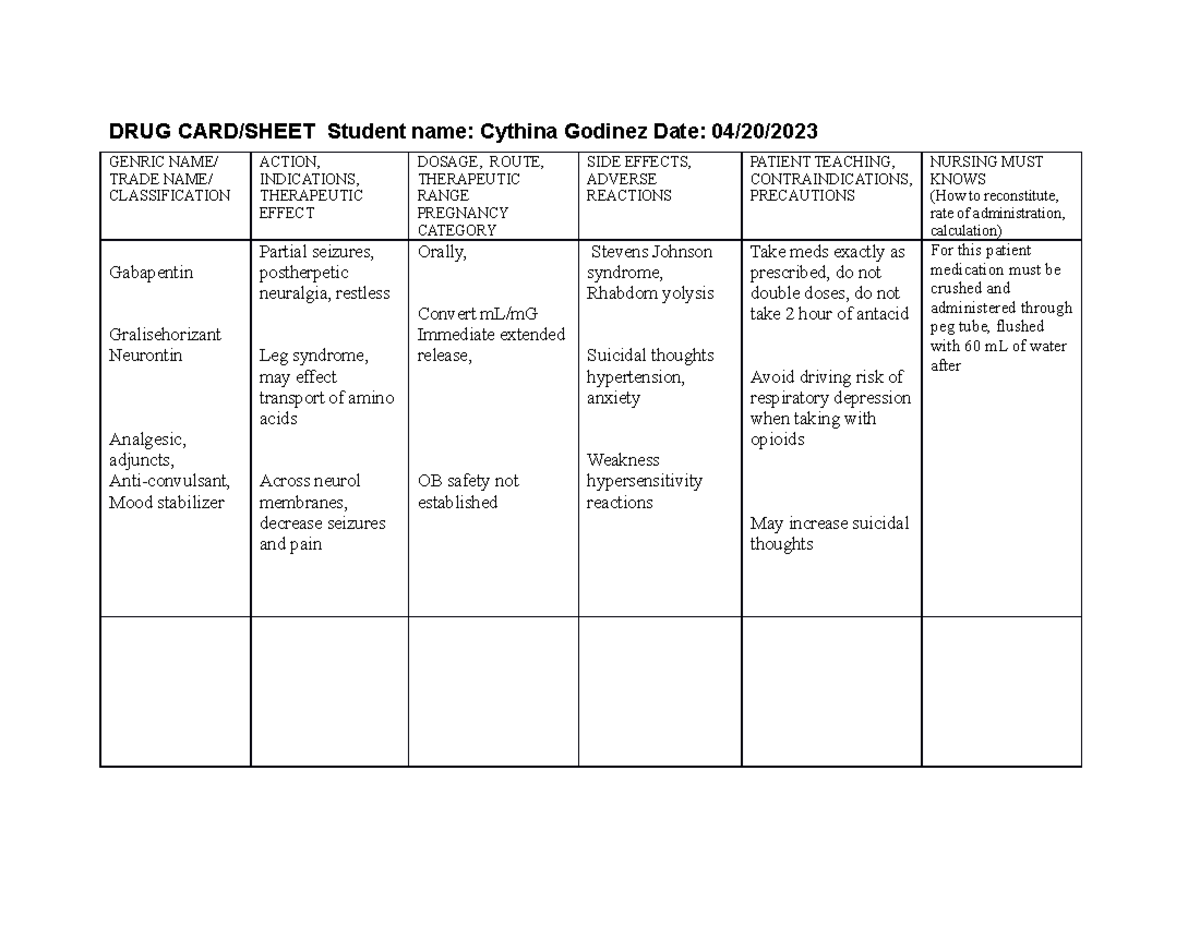 | 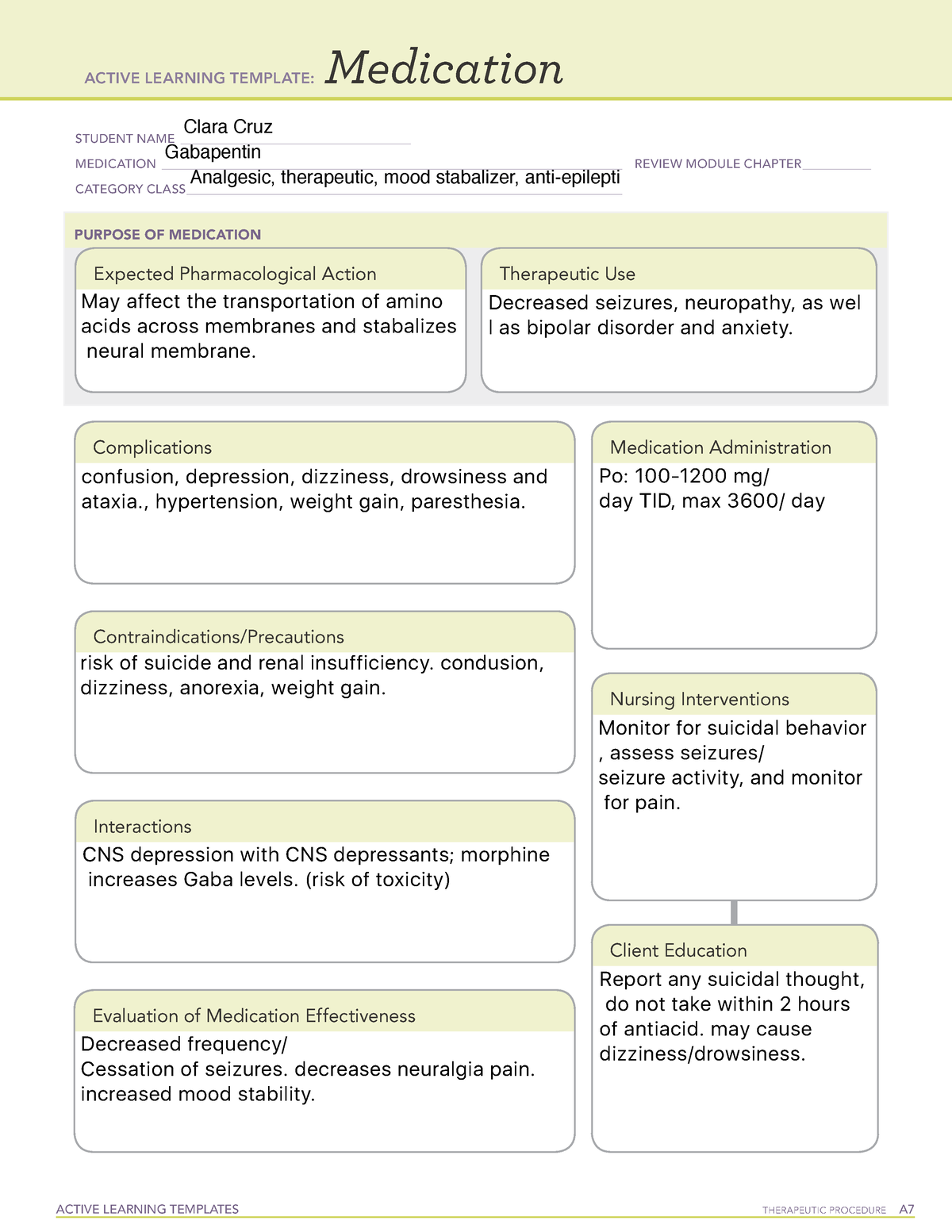 |
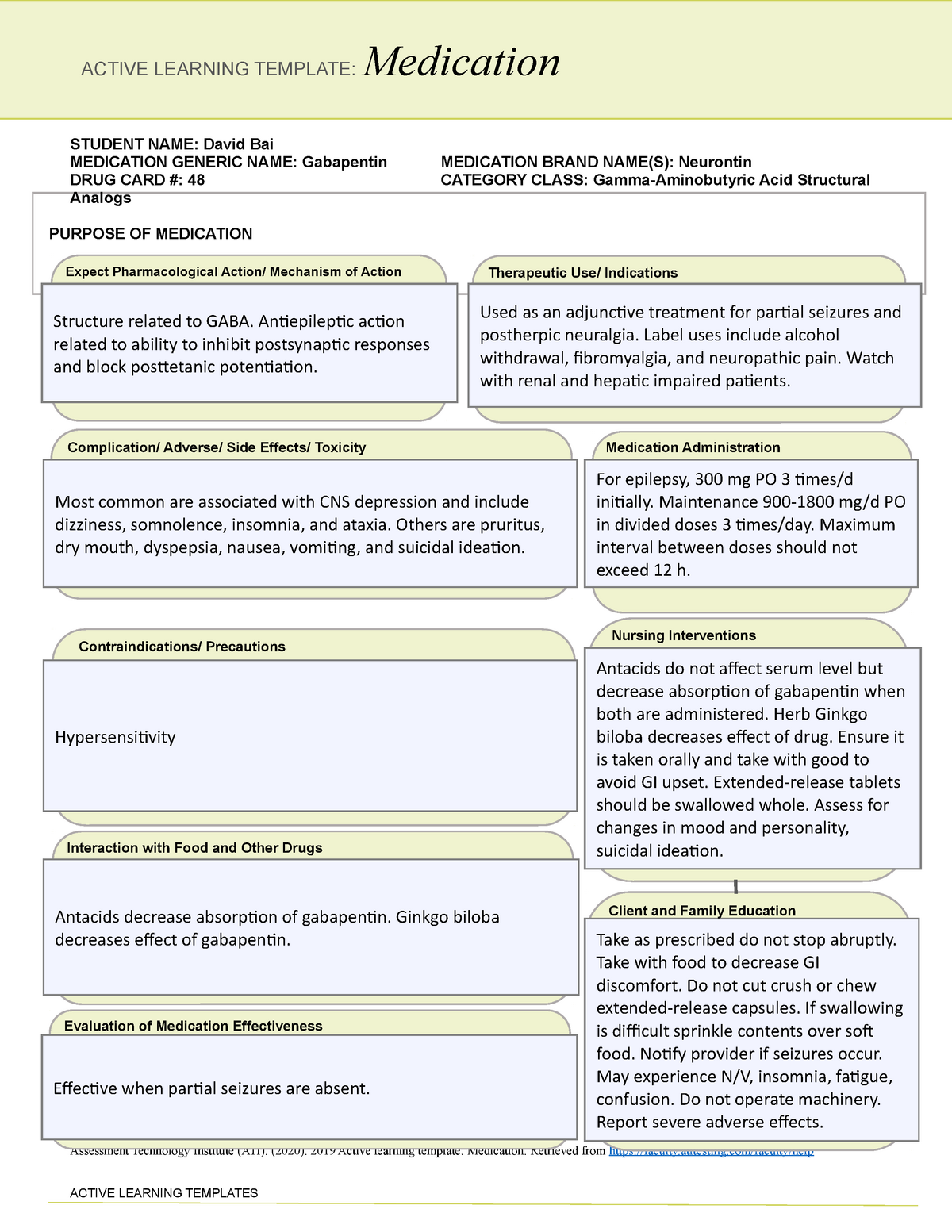
Gabapentinoids (e.g. pregabalin and gabapentin) are widely used in neurology, psychiatry and primary healthcare but are increasingly being reported as possessing a potential for misuse. In fact, increasing levels of both prescriptions and related fatalities, together with an anecdotally growing blac Some side effects of gabapentin may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Goff also said the board has started to track gabapentin sales. The U.S. Food and Drug Administration has approved gabapentin to treat seizures and pain caused by shingles. The U.S. Centers for Disease Control and Prevention has promoted the drug as a safer alternative to prescription opioids. Section 68-21-7 - Drugs of concern (a) Each of the following shall be classified as a drug of concern: (1) Any product containing all three of these drugs: butalbital, acetaminophen, and caffeine; (2) any compound, mixture, or preparation that contains any detectable quantity of ephedrine, its salts or optical isomers, or salts of optical isomers and is exempt from being reported to the Beginning that date, dispensers of gabapentin were required to begin reporting gabapentin to the PMP as a Schedule V controlled substance rather than a drug of concern. Schedule II drugs include highly addictive medications such as OxyContin and Vicodin, both potent opiates. Schedule III drugs include the lesser addictive drugs such as Tylenol We should consider introducing routine gabapentin testing in urine drug screens. This will inform clinical and political approaches to this possible new and dangerous type of substance misuse, as well as safe management of the distress caused by neuropathic pain. gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. K-TRACS requires reports of dispensations of controlled substances in schedules II-IV and drugs of concern outlined in K.A.R. 68-21-7: Any product containing all three of these drugs: butalbital, acetaminophen and caffeine At least 40% to 65% of individuals with prescriptions for gabapentin and roughly 20% for individuals who misuse opioids report gabapentin misuse. Gabapentin misuse may in part be driven by dependence and withdrawal symptoms. Gabapentin may cause breathing problems in people who use opioid pain medicines and those with chronic obstructive pulmonary disease (COPD). Older adults who take gabapentin also are at higher risk of breathing problems. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. Studies found that gabapentinoids are abused and misused and that individuals with a history of psychiatric disorders or substance use disorder seem to be at high risk. Moreover, some evidence supports the notion that patients with opioid use disorders may be at an increased risk of abusing gabapentinoids. Drugs of Concern—drugs other than controlled substances as defined by rule whose use requires tracking for public health purposes or which demonstrate a potential for abuse, including any material, compound, mixture, or preparation containing any In the US, gabapentin was approved by the Food and Drug Administration as a non-controlled substance. To date, and in spite of empirical evidence suggestive of diversion and abuse with opioids, gabapentin remains a non-controlled substance at the federal level. The following section describes these drugs of concern and their associated risks. WHAT IS DXM? DXM is a cough suppressor found in more than 120 over-the-counter (OTC) cold medications, either alone or in combination with other drugs such as analgesics (e.g., acetaminophen), antihistamines (e.g., chlorpheni-ramine), decongestants (e.g Gabapentin is a drug of concern, except when dispensed pursuant to a prescription issued by a veterinarian. G. “Patient ” means the ultimate user of a drug for whom a prescription is issued and for whom a drug is dispensed. requirement of two drugs of concern (promethazine in oral liquid formulations and gabapentin containing products) as of January 20, 2021. There is a continued downward trend in the number of opioid and controlled dangerous substance prescription dispensations to Louisiana patients. Hydrocodone bitartrate/acetaminophen remains the number one Gabapentin was primarily misused for recreational purposes, self-medication, or intentional self-harm and was misused alone or in combination with other substances, especially opioids, benzodiazepines, and/or alcohol. Individuals with histories of drug abuse were most often involved in its misuse. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. gabapentin is a safe drug to take, "however Gabapentin has known abuse potential and has been reported as an agent highly pursued for use in potentiating opioids.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | /cloudfront-us-east-1.images.arcpublishing.com/gray/3L6TKOMCWZF7PDRJD6UMPCYE2A.jpg) |
/cloudfront-us-east-1.images.arcpublishing.com/gray/NOI7MVPV3BAXNCFGKUR6SBWXFU.jpg) |  |
/cloudfront-us-east-1.images.arcpublishing.com/gray/VHHU3WK2VVHU3PWL43SCADA7TE.bmp) |  |
 |  |
 |  |