Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
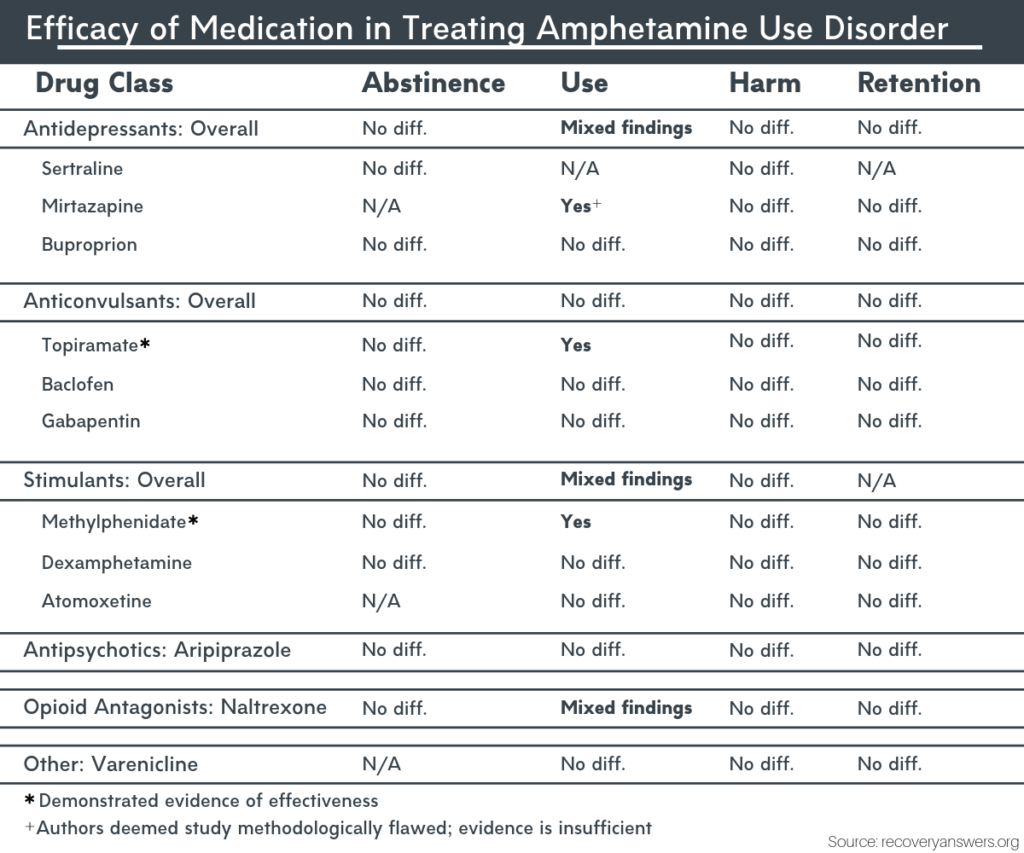
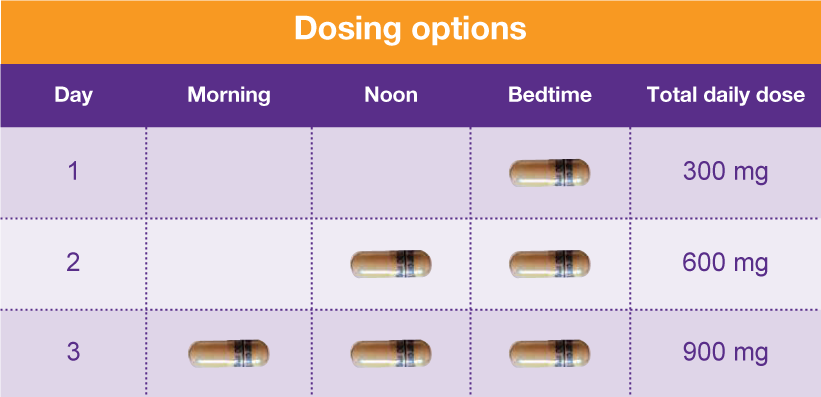
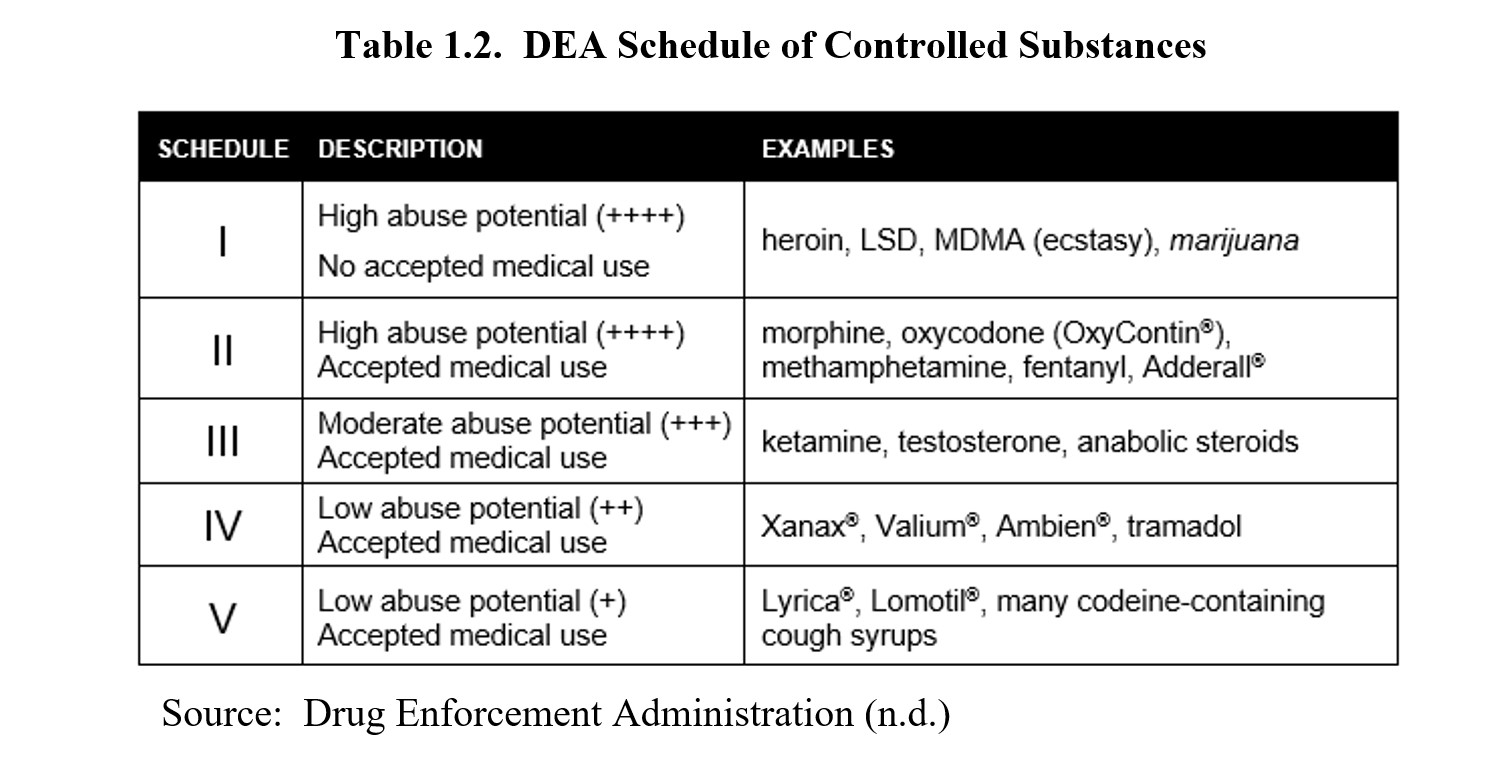
Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] The abuse of gabapentin and reclassification do not appear to be a local phenomenon based on the United Kingdom recently reclassifying gabapentin as a Schedule III controlled drug in April 2019. 27 The U.K. classification system is similar to the U.S. system in that there are 5 schedules, but it is slightly different in that the scheduling Find information on Gabapentin (Gralise, Horizant) in Davis’s Drug Guide including dosage, side effects, interactions, nursing implications, mechanism of action, half life, administration, and more. Davis Drug Guide PDF. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by Jennifer A. Shuford, M.D., M.P.H., Commissioner of Health, and Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Annual Review Completed for all Drug Entries on 9-15-2022 . Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. Abbreviations Used in Reference Table C.S.A Schedules I Schedule I II Schedule II III Schedule III Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Schedule III in KY (902 KAR 55:015). Schedule IV federally. Phenobarbital & noncontrolled active ingredient; 2285 III; N Quadrapax, Phenohydro, PB Hyos elixir; Schedule III in KY (schedule IV federally). Some products with no significant potential for abuse ; specifically exempted (see 902 KAR 55:045/CFR 1308.32). Link to current list In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). rug Administration for the treatment of neuropathic pain and epileptic disorders. In recent years, however, Gabapentin has been increasingly encountered by law enforcement, documented in national cr. me lab reports, reported to poison control centers, and diverted for illicit use. The Researched Abuse, Diversion and Addiction – R. Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with opioids and other drugs. Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen;
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |