Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
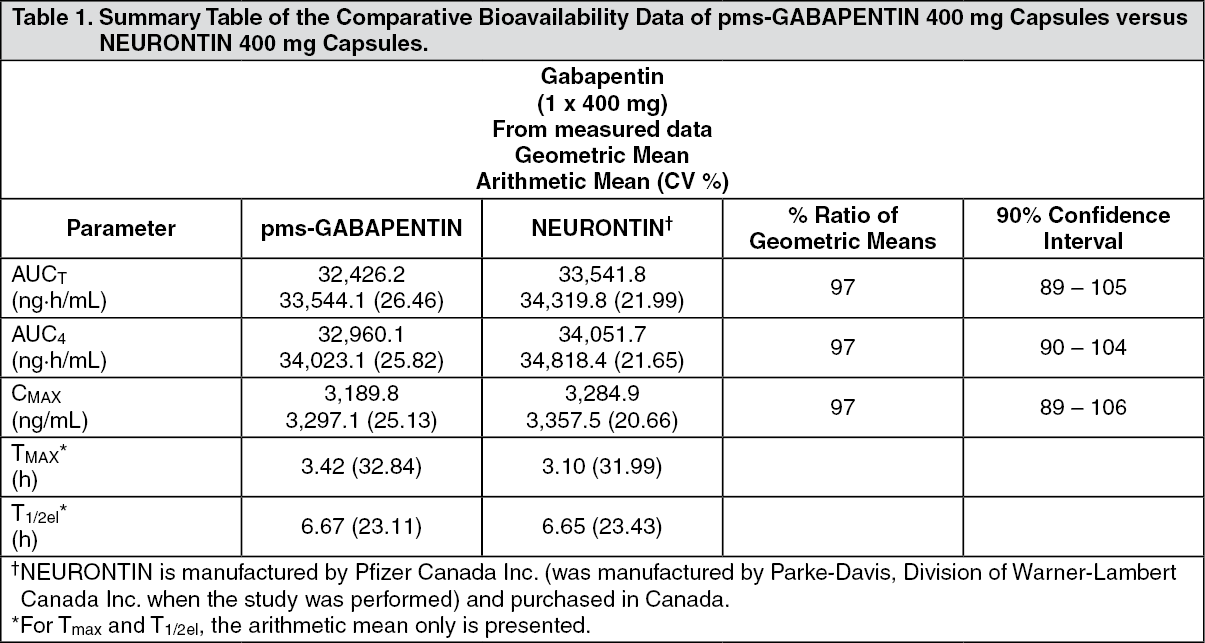
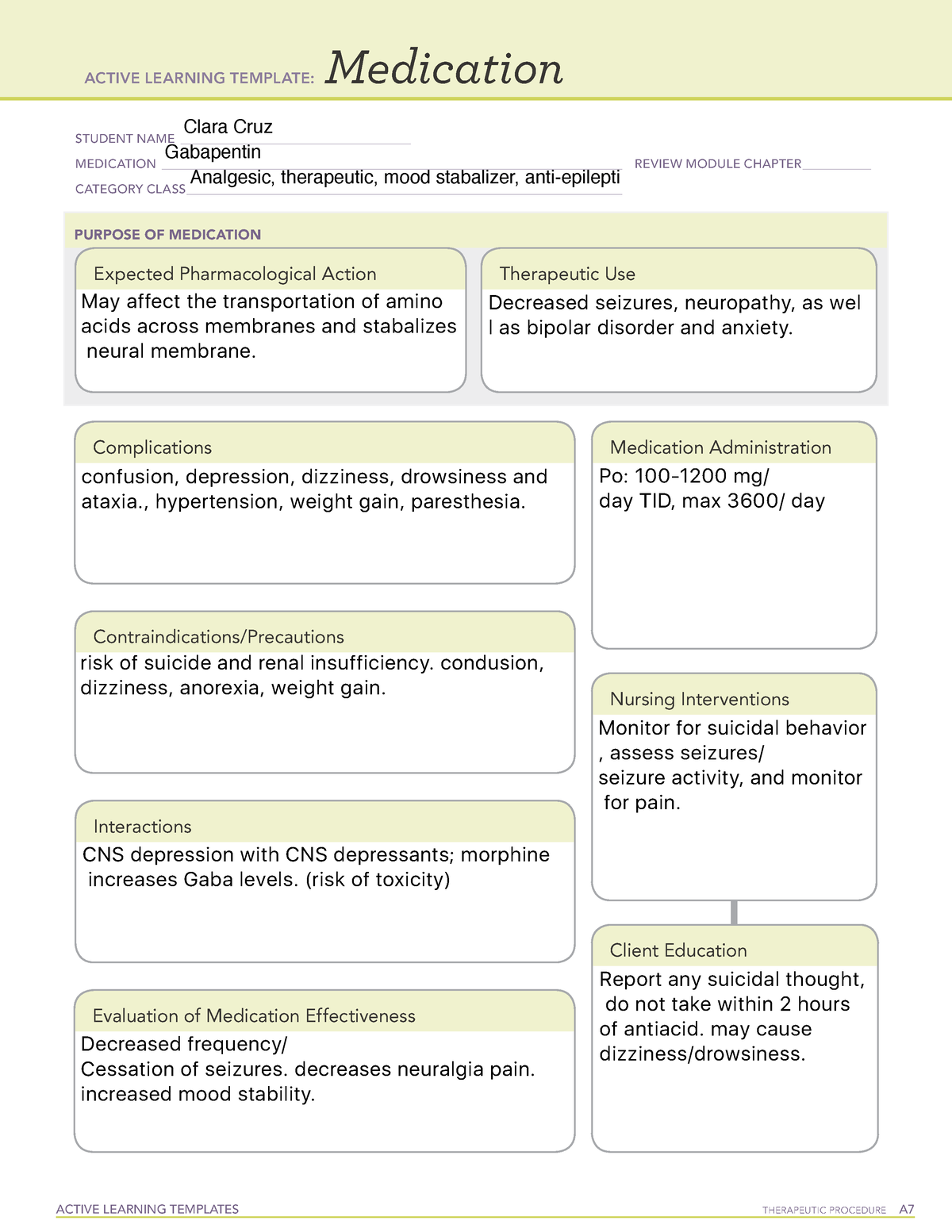
A 2017 systematic review of gabapentin for postherpetic neuralgia or diabetic neuropathy found gabapentin (1800 to 3600 mg daily) was more effective than placebo. 10 Evidence for gabapentin’s analgesic effect in other neuropathic pain conditions was very limited. 10 Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Clinical studies establishing efficacy of gabapentin for this indication were conducted with conventional (immediate-release) preparations; efficacy of gabapentin gastroretentive tablets and gabapentin enacarbil not established in patients with seizure disorders. Indication. In the United States, gabapentin is officially indicated for the treatment of postherpetic neuralgia in adults and for the adjunctive treatment of partial-onset seizures, with or without secondary generalization, in patients 3 years of age and older. 16 In Europe, gabapentin is indicated for adjunctive therapy in the treatment of The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The mechanisms of action are still unclear despite their widespread use. A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Indications and Usage for Gabapentin. • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. 2. Gabapentin Dosage and Administration. In a single (400 mg) and multiple dose (400 mg three times a day) study of GABARONE in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics. A second study compared primarily NEURONTIN 1200 mg/day, in three divided doses (N=101), with placebo (N=98). Additional smaller NEURONTIN dosage groups (600 mg/day, N=53; 1800 mg/day, N=54) were also studied for information regarding dose response. A third study compared gabapentin 900 mg/day, in three divided doses (N = 111) and placebo (N = 109). An additional gabapentin 1,200 mg/day dosage group (N = 52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Identify the appropriate indications for gabapentin therapy, including neuropathic pain, partial onset seizures, restless legs syndrome, and other relevant neurological and psychiatric conditions. In clinical studies, efficacy was demonstrated over a range of doses from 1800 mg/day to 3600 mg/day with comparable effects across the dose range; however, in these clinical studies, the Drug Name Generic Name : gabapentin Brand Name: Apo-Gabapentin (CAN), Gen-Gabapentin (CAN), Neurontin Classification: Antiepileptic Pregnancy Category C Dosage & Route Available forms : Capsules—100, 300, 400 mg; tablets—100, 300, 400, 600, 800 mg; oral solution—250 mg/5 mL ADULTS Epilepsy: Starting dose is 300 mg PO tid, then titrated up as needed. Maintenance: 900–1,800 mg/day PO in Use of the gabapentinoids for pain continues to increase. In 2018, the US Food and Drug Administration (FDA) strengthened the warnings for both gabapentin and pregabalin to emphasize the central nervous system side effects and the risk of respiratory depression, especially when combined with other centrally acting drugs. We reviewed the published comparative effectiveness literature for Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. We examined reporting practices for trials of gabapentin funded by Pfizer and Warner-Lambert's subsidiary, Parke-Davis (hereafter referred to as Pfizer and Parke-Davis) for off-label indications Gabapentin is a medication commonly prescribed to treat various conditions, including epilepsy, neuropathic pain, and restless legs syndrome. This guide aims to educate patients about important considerations, including dosage instructions, potential side effects, and precautions, to ensure safe and effective use of gabapentin. However, over the past 25 years, gabapentin has been prescribed for a variety of indications beyond those formally evaluated by the FDA. In this issue of JAMA, Moore et al 1 summarize an updated Cochrane review on the use of gabapentin for neuropathic pain. HORIZANT is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. As a prodrug of gabapentin, HORIZANT also increases this risk. Gabapentin is prescribed for seizures and pain and has efficacy for treating alcohol use disorder (AUD) starting at doses of 900 milligrams per day (mg/d). Recent evidence suggests safety concerns associated with gabapentin including adverse neurologic effects.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |