Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
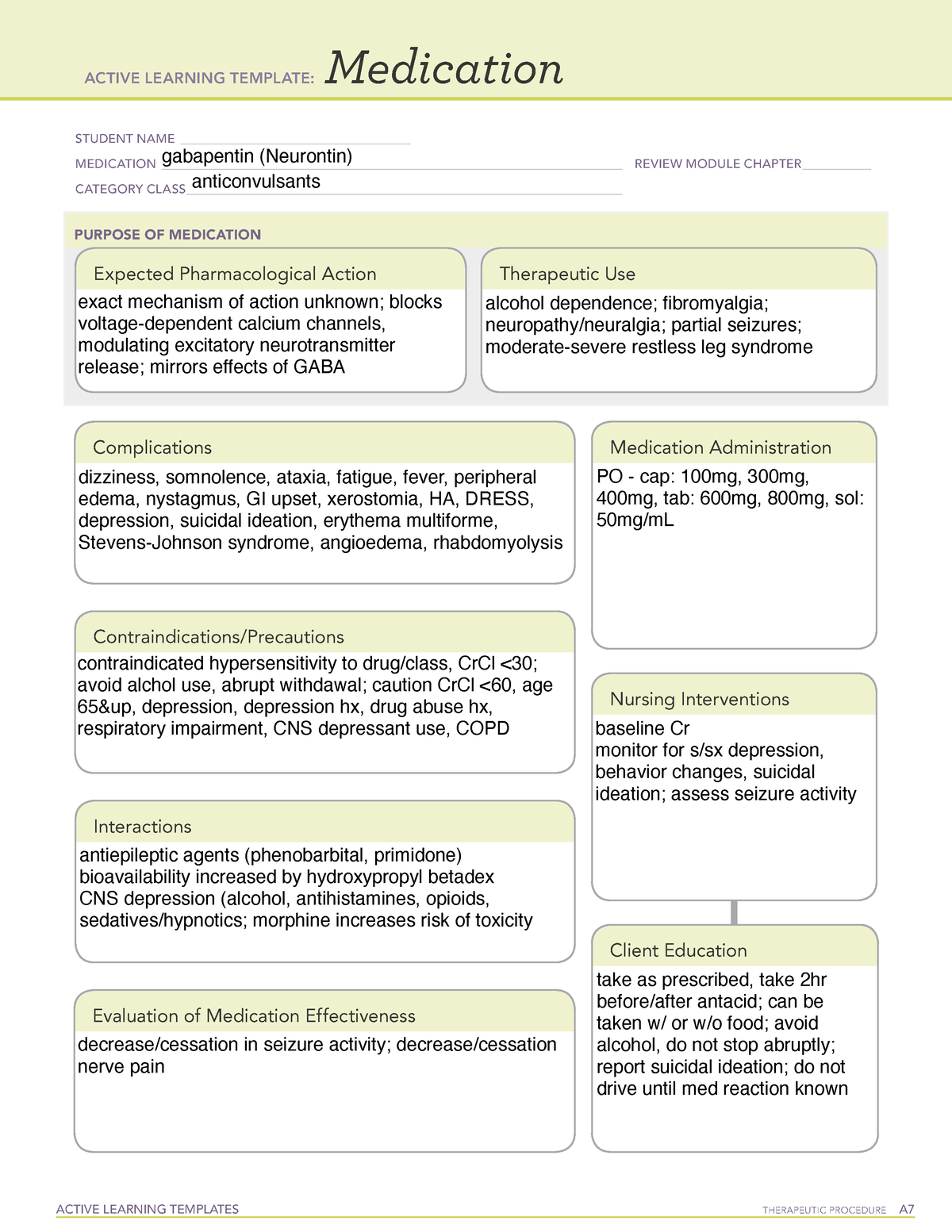
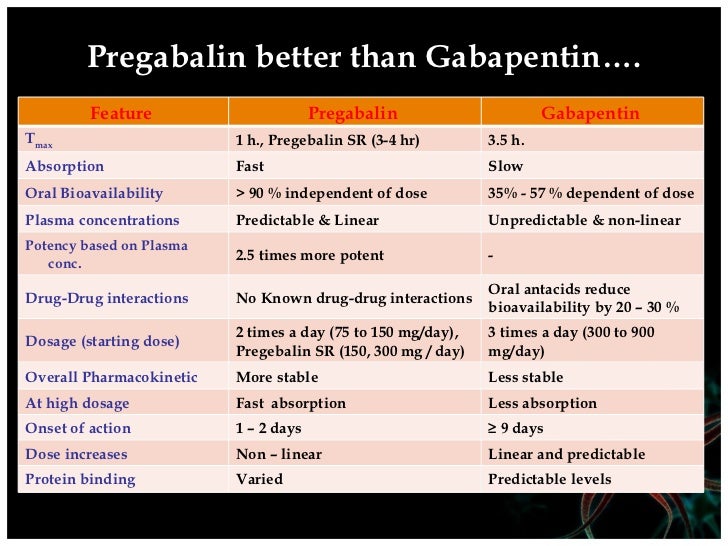
DG01245 Gabapentin D09539 Gabapentin enacarbil Drug classes [BR:br08332] Neuropsychiatric agent DG03199 Antiepileptic agent D09539 Gabapentin enacarbil Target-based classification of drugs [BR:br08310] Ion channels Voltage-gated ion channels Calcium channels CACNA2D D09539 Gabapentin enacarbil (JAN/USAN/INN) <JP/US> Transporters Solute carrier The oral bioavailability of gabapentin enacarbil (as gabapentin) is greater than or equal to 68%, across all doses assessed (up to 2,800 mg), with a mean of approximately 75%. [25] [1] In contrast to the other gabapentinoids, the pharmacokinetics of phenibut have been little-studied, and its oral bioavailability is unknown. [28] Common Generic Name(s): gabapentin enacarbil; Pronunciation: GAB-a-pen-tin, ho-RI-zant; Drug Classes: GABA analog; Availability: prescription only, no generic available; How is it used? HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for Drug Name Active Ingred Mfr Indication Approval Date AHFS Class NDA Chem Type* Appl No; Natroba ® spinosad: Parapro: head lice infestations: 1/18/2011: 84:04.12 - Scabicides and Pediculicides : Viibryd ® vilazodone: Forest: major depressive disorder: 1/21/2011: 28:16.04.24 - Serotonin Modulators : Edarbi ® azilsartan: Takeda: hypertension: 2 Gabapentin enacarbil, the prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Elimination Route Excreted renally as unchanged drug. Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Drug Substance: Gabapentin enacarbil (also called XP 13512) is a non-ester, pro-drug of gabapentin (a marketed drug). It has a molecular formula C16H27NO6 and molecular weight 329.39. There is a single chiral center , the drug substance is a racemic mixture. Gabapentin enacarbil is a white to off-white powder, soluble in (b) (4) (b) (4) (b) (4) Compare Gabapentin vs Gabapentin Enacarbil head-to-head with other drugs for uses, ratings, cost, side effects and interactions. Gabapentin enacarbil (Horizant (ER) (U.S. Tooltip United States), Regnite (in Japan)) is an anticonvulsant and analgesic drug of the gabapentinoid class, and a prodrug to gabapentin. [1] Important Safety Information for HORIZANT® (gabapentin enacarbil) Extended-Release Tablets INDICATIONS. HORIZANT® (gabapentin enacarbil) is a prescription medicine used to: treat adults with moderate to severe primary Restless Legs Syndrome (RLS). Gabapentin Enacarbil is a prescription medication used for treating the symptoms of restless leg syndrome and postherpetic neuralgia. Gabapentin Enacarbil is available under the following different brand names: Horizant Detailed drug Information for Gabapentin enacarbil. Includes common brand names, drug descriptions, warnings, side effects and dosing information. Horizant (gabapentin enacarbil) is an anticonvulsant drug prescribed for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. Horizant is not recommended for patients who are required to sleep during the daytime and remain awake at night. GABAPENTIN ENACARBIL is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2011 and is indicated for neuralgia and restless legs syndrome and has 4 investigational indications. Horizant (gabapentin enacarbil) is used to treat restless legs syndrome and nerve pain caused by the herpes virus. Includes Horizant side effects, interactions and indications. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Horizant (gabapentin enacarbil) is FDA-approved to treat restless leg syndrome (RLS) and nerve pain from shingles in adults. This medication belongs to the drug class called antiepileptics. Horizant (gabapentin enacarbil) is an extended-release tablet that's taken by mouth. Gabapentin enacarbil is a gabapentin prodrug used to treat Restless Legs Syndrome (RLS) and postherpetic neuralgia (PHN). Gabapentin enacarbil extended release tablets available under the trade name Horizant and gabapentin are not interchangeable. Use: Postherpetic neuralgia. Usual Adult Dose for Restless Legs Syndrome: Gabapentin enacarbil available under the trade name Horizant: 600 mg orally once daily with food at about 5 PM
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |