Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
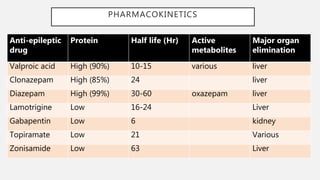 |  |
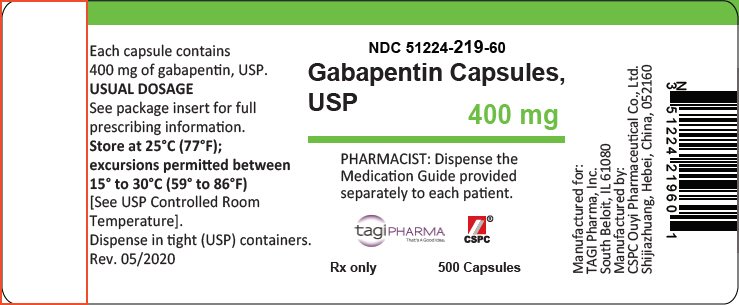
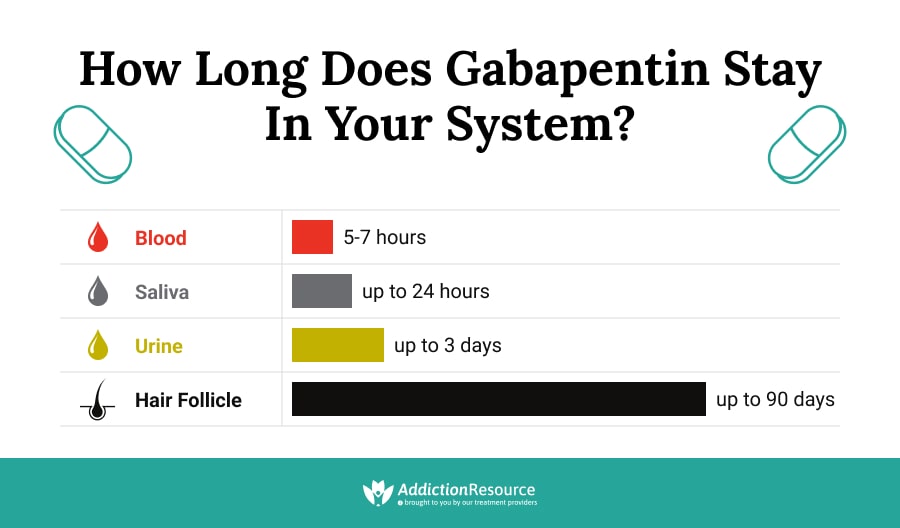
The elimination half-life (t 1/2) of gabapentin ranges from 5.1 to 6.0 hours and is unaltered by dose or following multiple doses of HORIZANT. Specific Populations Race The short half-life of gabapentin in vivo ranges from 5 to 7 hours requiring the patient to take 3 to 4 doses per day in order to provide therapeutic concentrations. GEn was engineered to overcome these variances in absorption. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In patients with Cl cr <30 mL/minute, half-life of 52 hours reported with conventional (immediate-release) gabapentin; in anuric patients, half-life reported to be 132 hours on nondialysis days and 3.8 hours during hemodialysis. (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. 1.2 . Management of Postherpetic Neuralgia Gabapentin enacarbil is eliminated primarily in the urine (94%) and to a lesser extent in the feces (5%). The elimination half-life of gabapentin is 5.1 to 6.0 hours. With structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. Includes Gabapentin Enacarbil indications, dosage/administration, pharmacology, mechanism/onset/duration of action, half-life, dosage forms, interactions, warnings, adverse reactions, off-label uses and more. The sequence of the treatments was fixed in the study, so that each subject was titrated with the same drug (GBP-IR) and tapered with the same drug (GEn). Also, given the half-life of gabapentin is 5 – 7 hours, each treatment was predicted to reach steady state by day 5, the day of PK measurements. 7.2 Gabapentin and pregabalin. The gabapentinoids are medications that do not appear to bind to sodium channels in either neural or cardiac tissue. The effects of gabapentin enacarbil (GBPe), a prodrug of gabapentin (GBP), on cardiac repolarization were investigated in a single-center, double-blind, randomized, placebo-controlled, escalating-dose, crossover trial in 32 healthy volunteers who Gabapentin enacarbil (Horizant (ER) (U.S. Tooltip United States), Regnite (in Japan)) is an anticonvulsant and analgesic drug of the gabapentinoid class, and a prodrug to gabapentin. [1] Therefore, gabapentin enacarbil (XXX), a carbamate conjugate of gabapentin, has been designed with improved bioavailability, absorption, and half-life which undergoes hydrolysis by nonspecific esterase (particularly in enterocytes) releasing the active drug gabapentin [53]. This conjugate is also responsible for absorption of essential Horizant® (gabapentin enacarbil) offers once daily dosing for RLS and single step titration for PHN. Learn how to dose HORIZANT®. See Important Safety Information. Neurontin has a relatively short half-life and duration of action. The reported half-life (the time it takes for 50% of the drug to be metabolized) is 5 to 7 hours, which necessitates a dosing frequency of 3 to 4 times daily for it to be effective. Most studies report that gabapentin has a duration of action of 6 to 8 hours. Elimination half-life: o 5.1 to 6 hrs Table 1 - Pharmacokinetic parameters of GEn and gabapentin. 1, 3 5 . Parameter Gabapentin Enacarbil Gabapentin . Bioavailability (%) Fasting 600 mg: 42 to 65 900 mg: 60 1200 mg: 47 2400 mg: 34 3600 mg: 33 4800 mg: 27 Fed 75 Increase 14% AUC Tmax (hr) Fasting 5 2.7 to 3.3 Fed 7.3 N/A Gabapentin has been shown to improve RLS in a small number of clinical studies, but is limited by its short half-life and variable bioavailability. Gabapentin enacarbil is a novel prodrug of gabapentin designed to overcome these pharmacokinetic limitations. Compare Gabapentin vs Gabapentin Enacarbil head-to-head with other drugs for uses, ratings, cost, side effects and interactions. Gabapentin enacarbil was approved by the Food and Drug Administration (FDA) in April of 2011. This article reviews clinically significant aspects of this new drug including: the FDA-approved indications, mechanism of action, administration, drug interactions, adverse effects, clinical trial evidence, innovative properties and place in therapy. Gabapentin Pregabalin Gabapentin enacarbil; Time to maximum blood level: 2 h: 1.5 h: 7-9 h: Elimination half-life: 5-7 h: 6 h: Relatively stable plasma levels during 18-24 h (elimination half-life, 6 h) Metabolism and excretion: Renal: Renal: Intestinal metabolism; renal excretion: Initial daily dose: 300 mg a: 75 mg a: 600 mg a: Maximum daily GBP is quickly excreted unaltered by the kidneys within the urine possessing a half-life ranging between five and seven hours. 1 A limited half-life alongside an unpredictable bioavailability restricts use. 3 Regular dosing is thus required to maintain beneficial concentrations. gabapentin enacarbil decreases levels of levocarnitine by unspecified interaction mechanism. Minor/Significance Unknown. pancuronium. gabapentin enacarbil decreases effects of pancuronium by pharmacodynamic antagonism. Minor/Significance Unknown. rapacuronium. gabapentin enacarbil decreases effects of rapacuronium by pharmacodynamic antagonism.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |