Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
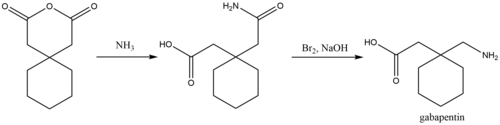
Gabapentin shows negligible binding to serum proteins and undergoes little or no metabolism [87]. The main clearance is by the kidneys. Patients with renal failure show a prolonged elimination half-life. Hemodialysis effectively clears gabapentin from the circulation [13]. Metabolism. Gabapentin is not appreciably metabolized. Gabapentin enacarbil, the prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Elimination Route. Excreted renally as unchanged drug. Half-life. Approximately 5–7 hours. Special Populations Gabapentin enacarbil is absorbed in the intestines by active transport through the proton-linked monocarboxylate transporter, MCT-1. Gabapentin Enacarbil: A Review in Restless Legs . Syndrome. Drugs 2016; 76: hypersensitivity and suppresses medial prefrontal cortical glucose metabolism . in rats with neuropathic pain. Mol Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that provides sustained dose-proportional exposure to gabapentin and predictable bioavailability. Gabapentin enacarbil is approved by the US Food and Drug Administration for the treatment of moderate-to-severe primary restle Gabapentin enacarbil (Horizant®) is a prodrug of gabapentin which binds with high affinity to the α2δ subunit of voltage-activated calcium channels in vitro studies. It is unknown how the binding of gabapentin enacarbil (GEn) to the α2δ subunit corresponds to the treatment of restless leg syndrome (RLS) symptoms. Objective: Gabapentin immediate release (GBP-IR), gabapentin gastric retentive (GBP-GR), and the prodrug gabapentin enacarbil extended release formulation (GEn) have been approved for management of postherpetic neuralgia (PHN) in adults. This is the first pharmacokinetic (PK) comparison of all three formulations using FDA-recommended doses for PHN. Gabapentin enacarbil, a prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Unlike gabapentin, gabapentin enacarbil is absorbed via high-capacity transporters throughout the GI tract and is not affected by saturable absorption; this improves bioavailability of the Non-dopaminergic drugs such as oral gabapentin (GBP) have been more recently advocated. Despite ameliorating RLS symptoms, GBP’s pharmacokinetic limitations restrict its overall effectiveness. A novel specifically designed prodrug, gabapentin enacarbil (GE), has demonstrated successful RLS alleviation with a superior pharmacokinetic profile. Gabapentin is cleared via renal excretion, and its elimination is proportional to creatinine clearance (CrCL); CrCL can, therefore, be used as a predictor of gabapentin renal clearance. Gabapentin produced from hydrolysis of gabapentin enacarbil is also eliminated via the renal clearance pathway. Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that provides sustained dose-proportional exposure to gabapentin and predictable bioavailability. Gabapentin enacarbil is Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. gabapentin enacarbil decreases levels of cyanocobalamin by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown. dexmethylphenidate. dexmethylphenidate increases effects of gabapentin enacarbil by decreasing metabolism. Minor/Significance Unknown. levocarnitine Gabapentin enacarbil is a prodrug of gabapentin (Neurontin®, Pfizer) which binds to the α2-δ subunit of L-type voltage-regulated calcium channels, reducing the release of several neurotransmitters. 122,123 Gabapentin enacarbil was discovered at XenoPort, co-developed with GlaxoSmithKline, is marketed under the brand name Horizant®, and is approved for the treatment of moderate to severe Gabapentin enacarbil, a transported acyloxyalkylcarbamate prodrug of gabapentin, provides predictable and dose-proportional gabapentin exposure (AUC). Gabapentin is cleared via renal excretion, and its elimination is proportional to creatinine clearance (CrCL); CrCL can, therefore, be used as a predictor of gabapentin renal clearance. Gabapentin enacarbil is hydrolyzed to gabapentin in the gastrointestinal tract. Gabapentin itself undergoes little subsequent metabolism and is excreted in the urine unchanged. Gabapentin and gabapentin enacarbil do not induce or inhibit the drug metabolizing, microsomal cytochrome P450 enzymes. Gabapentin enacarbil is absorbed in the intestines by active transport through the proton-linked monocarboxylate transporter, MCT-1. Volume of distribution. The volume of distribution is 76L. Protein binding. Gabapentin plasma protein binding is less than 3%. Metabolism. Gabapentin enacarbil does not interact with any of the major cytochrome Gabapentin enacarbil is converted to gabapentin during absorption, before reaching the systemic circulation. 16 Gabapentin produced by hydrolysis of gabapentin enacarbil is also eliminated by the renal clearance pathway, without further metabolism.16, 17 In patients with normal CrCL (≥60 mL/min), CL R after oral administration of gabapentin A randomized placebo-controlled trial evaluated gabapentin enacarbil for migraine prophylaxis [35 C]. A total of 526 patients were randomized to receive gabapentin enacarbil 1200, 1800, 2400, 3000 mg, or placebo daily. Use of gabapentin enacarbil did not differ significantly from placebo in headache prophylaxis.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |