Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
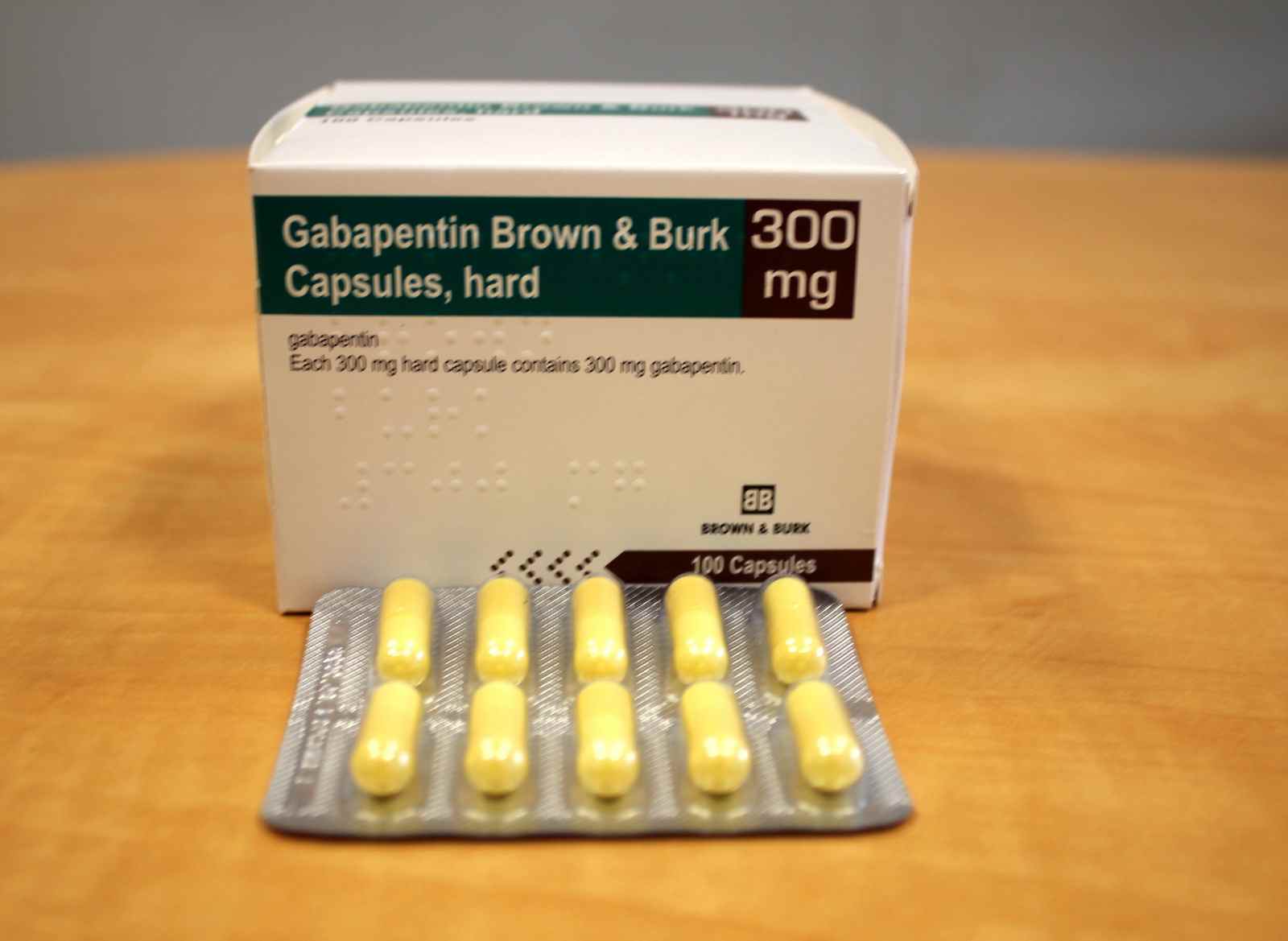
Gabapentin enacarbil extended release tablets available under the trade name Horizant and gabapentin are not interchangeable. Use: Postherpetic neuralgia. Usual Adult Dose of Horizant for Restless Legs Syndrome: 600 mg orally once daily with food at about 5 PM. Generic name: gabapentin systemic. double-blind, placebo-controlled study support the use of extended-release gabapentin for decreasing frequency and severity of Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older Gralise (gabapentin) is FDA-approved for treating nerve pain from shingles in adults. It belongs to the drug class called antiepileptics.Gralise (gabapentin) is an extended-release form of gabapentin, which means that the medication gets slowly released into the body from the tablet. Causes rapid release of drug from gabapentin enacarbil extended-release tablets. Avoid concomitant use. Antacids. Reduced bioavailability of gabapentin, but to a smaller extent when gabapentin administered 2 hours after antacid. Administer gabapentin at least 2 hours after antacid. Anticonvulsants Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Common Generic Name(s): gabapentin enacarbil; Pronunciation: GAB-a-pen-tin, ho-RI-zant; Drug Classes: GABA analog; Availability: prescription only, no generic available; How is it used? tablet Gabapentin is also available as an extended-release tablet that works for a longer length of time; this is the only formulation that is approved for restless legs syndrome. Gabapentin is taken by mouth and comes in capsule, tablet, and liquid form. Generic Name: gabapentin enacarbil extended-release tablets; Brand Name: Horizant; Drug Class: GABA Analogs Gabapentin is a medication that treats many medical conditions. It comes as an immediate-release (IR) form and two extended-release (ER) forms called Horizant and Gralise. Horizant and Gralise were FDA-approved in 2011. These medications are longer-acting than IR gabapentin and don’t have to be taken as often throughout the day. Expert Opin Drug Deliv. 2012;9(9):1147-1160. Chen C, Cowles VE, Hou E. Pharmacokinetics of gabapentin in a novel gastric-retentive extended-release formulation: comparison with an immediate-release formulation and effect of dose escalation and food. J Clin Pharmacol. 2011;51(3):346-358. Immediate-release forms of gabapentin (Neurontin, generic gabapentin) can be taken with or without food. The longer-acting forms of gabapentin (Horizant, Gralise) should be taken with food or a meal to help improve the absorption of the medicine. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the management of postherpetic neuralgia (PHN GABAPENTIN (GA ba pen tin) treats nerve pain. It works by calming overactive nerves in your body. HORIZANT is an extended-release formulation of gabapentin enacarbil, a prodrug of gabapentin. HORIZANT provides approximately dose-proportional and extended exposure to gabapentin over the range 300 to 6,000 mg. HORIZANT and gabapentin are not interchangeable because the same daily dose of each results in different plasma concentrations of Generic Horizant Availability. Last updated on Mar 13, 2025. Horizant is a brand name of gabapentin enacarbil, approved by the FDA in the following formulation(s): HORIZANT (gabapentin enacarbil - tablet, extended release;oral) Manufacturer: AZURITY Approval date: April 6, 2011 Strength(s): 600MG ; Manufacturer: AZURITY Approval date: December
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |