Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
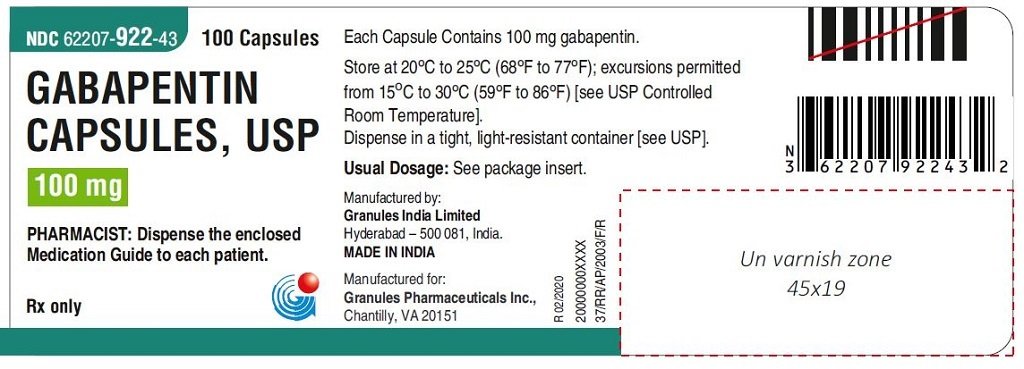
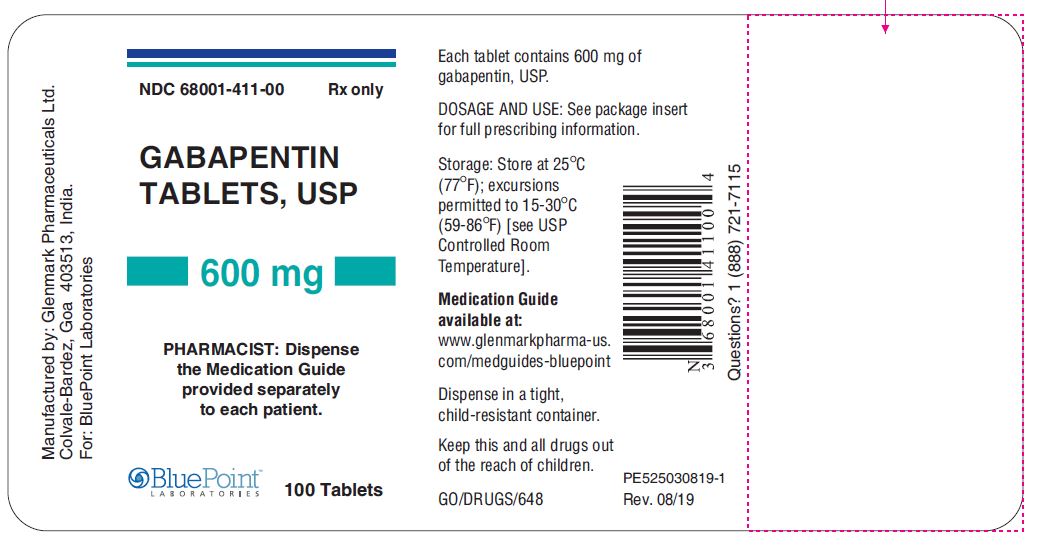
Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin . 7.2 Carbamazepine . This label may not be the latest approved by FDA. For current labeling information, please In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures. 9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Neurontin for pain. 4 By the mid FDA approved labeling text (dated 10/12/00) Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. FDA Approved Labeling Text dated 05/01/2013 Page 4 of 37 . gabapentin dose may be required in patients who have age related compromised renal function. (See . PRECAUTIONS, Geriatric Use, and . DOSAGE AND ADMINISTRATION.) Pediatric: Gabapentin pharmacokinetics were determined in 48 pediatric subjects between the The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). The FDA product label includes the following information: 1 indications and usage, 2.1 dosage for postherpetic neuralgia, 2.2 dosage for epilepsy with partial onset Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. This label may not be the latest approved by FDA. For current labeling information, please visit 11-point numeric pain rating scale ranging from 0 (no The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- NDA 022399 – FDA Approved Labeling Text dated December 2012 . HIGHLIGHTS OF PRESCRIBING INFORMATION . These highlights do not include all the information needed to use HORIZANT safely and effectively. See full prescribing information for HORIZANT. HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use Initial U.S. Approval: 2011 Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was 6.2 Adverse Events Associated With Gabapentin . 7 DRUG INTERACTIONS This label may not be the latest approved by FDA. For current labeling information, please Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly FDA Approved Labeling Text dated 03/01/2011 Page 2 . particular, gabapentin prevents pain-related responses in several models of neuropathic pain in rats or mice (e.g. spinal nerve ligation To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication 45 Antiepileptic drugs (AEDs), including gabapentin, the active ingredient in GRALISE, 46 increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |