Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
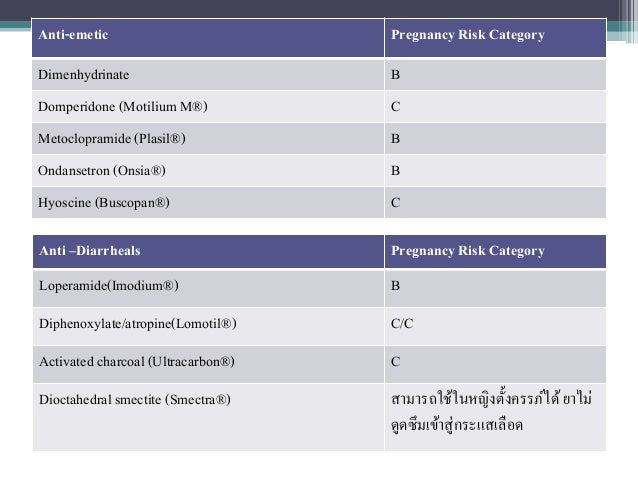 | 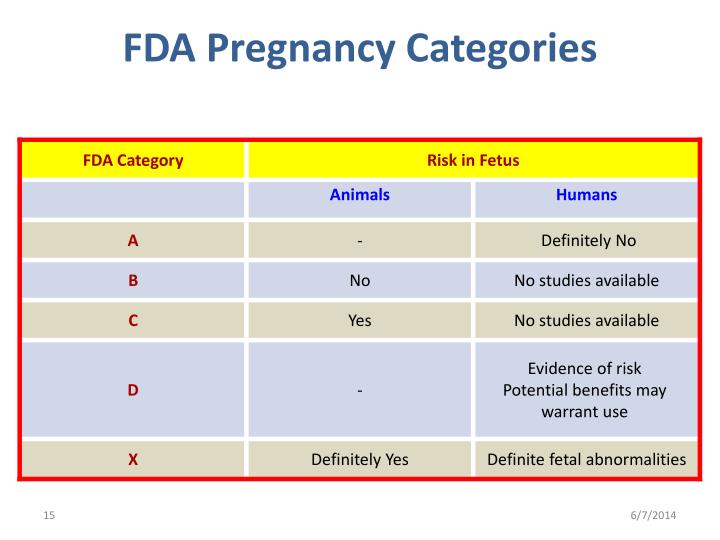 |
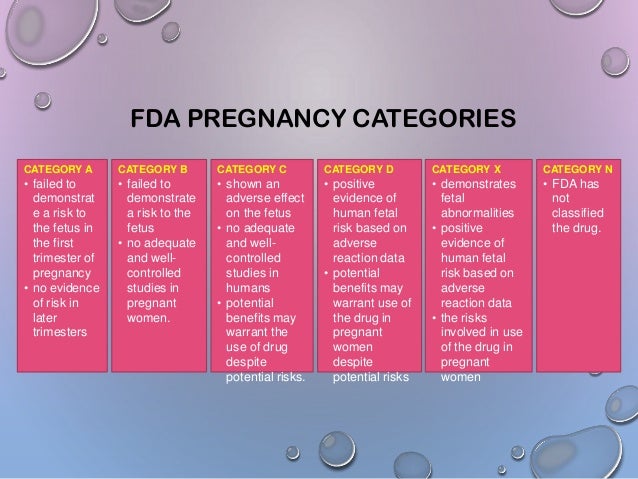
The Pregnancy subsection (8.1) includes information for a pregnancy exposure registry for the drug when one is available. Pregnancy exposure registries collect and maintain data on the effects of We identified 4,642 pregnancies exposed in T1 (mean age = 28 years; 69% white), 3,745 exposed in early pregnancy only (28 years; 67% white), 556 exposed in late pregnancy only (27 years; 60% white), and 1,275 exposed in both early and late pregnancy (29 years; 75% white). Overview of the five pregnancy risk categories, established by the FDA to indicate the potential of a drug to cause birth defects if used during pregnancy. นิยามศัพท์. Pregnancy Category. A: จากการศึกษาไม่พบความเสี่ยงต่อทารกในครรภ์ทั้งในไตรมาส 1และ 3 ยาที่จัดอยู่ในระดับนี้แทบไม่มีอันตรายต่อทารกในครรภ์ Drug FDA pregnancy category* AAP rating Lactation risk category†; Anxiolytics and hypnotics: Benzodiazepines: Alprazolam (Xanax) D: Unknown, of concern: L3: Chlordiazepoxide (Librium) The U.S. Food and Drug Administration classifies gabapentin (Neurontin) as a Pregnancy Category C medication, which means that animal studies conducted on this medication has caused harm on the fetus. Advice and warnings for the use of Gabapentin during pregnancy. FDA Pregnancy Category C - Risk cannot be ruled out Is gabapentin dangerous in pregnancy? Because the risks of taking gabapentin while pregnant in humans are not fully understood, use of gabapentin during pregnancy is determined on a case-by-case basis to determine if the benefits outweigh the risks. Gabapentin is a pregnancy category C, which means risk cannot be ruled out. the underlying information that informed the assignment of the pregnancy category. FDA believes that a narrative structure for pregnancy labeling, rather than a category system, is best able to Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. gabapentin dose may be required in patients who have age related compromised renal function. (See PRECAUTIONS, Geriatric Use, and DOSAGE AND ADMINISTRATION.) Pediatric: We identified 4,642 pregnancies exposed in T1 (mean age = 28 years; 69% white), 3,745 exposed in early pregnancy only (28 years; 67% white), 556 exposed in late pregnancy only (27 years; 60% white), and 1,275 exposed in both early and late pregnancy (29 years; 75% white). This article summarizes the current literature regarding gabapentin use during pregnancy and related prenatal and neonatal exposure outcomes with special consideration for interactions between gabapentin and opioid use. US FDA pregnancy category Not Assigned: The US FDA has amended the pregnancy labeling rule for prescription drug products to require labeling that includes a summary of risk, a discussion of the data supporting that summary, and relevant information to help health care providers make prescribing decisions and counsel women about the use of During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or The objective of this study was to assess the safety of gabapentin (Neurontin) exposure in human pregnancy. Prospective and retrospective data concerning 51 fetuses, including 3 twin gestations, were collected from 39 women with epilepsy and other disorders exposed to gabapentin during pregnancy. 5.3 Withdrawal of Gabapentin 5.4 . Tumorigenic Potential 5.5 . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.6. Laboratory Tests 6 ADVERSE REACTIONS . 6.1 Clinical Trials Experience . 6.2 Postmarketing and Other Experience with other Formulations of Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |