Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
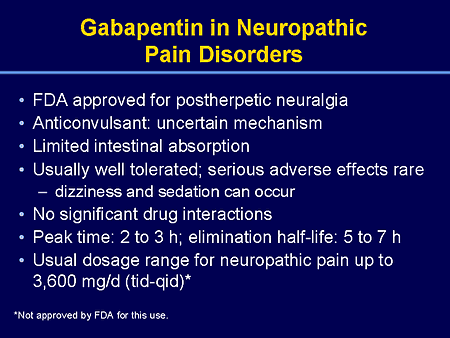 |  |
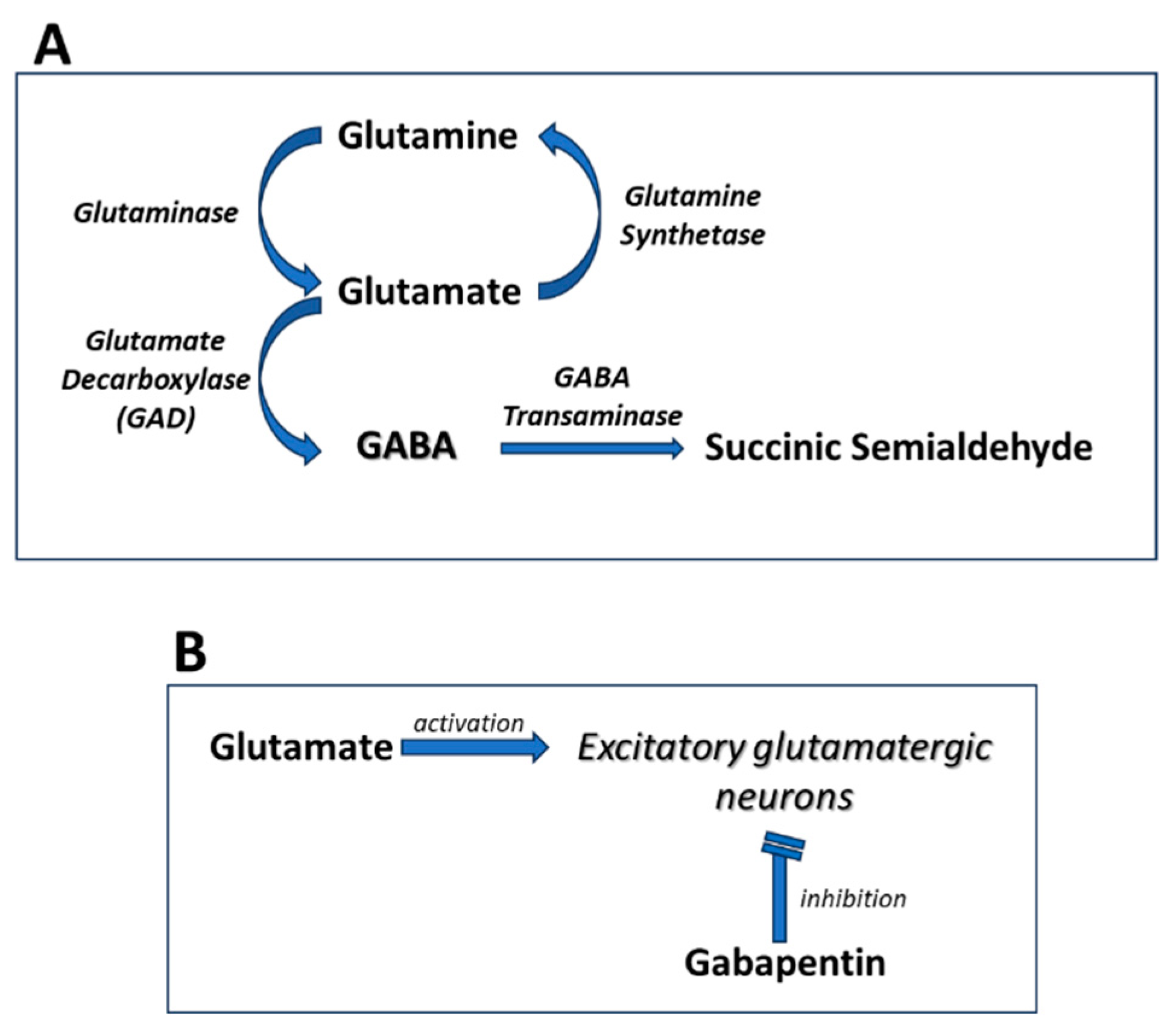
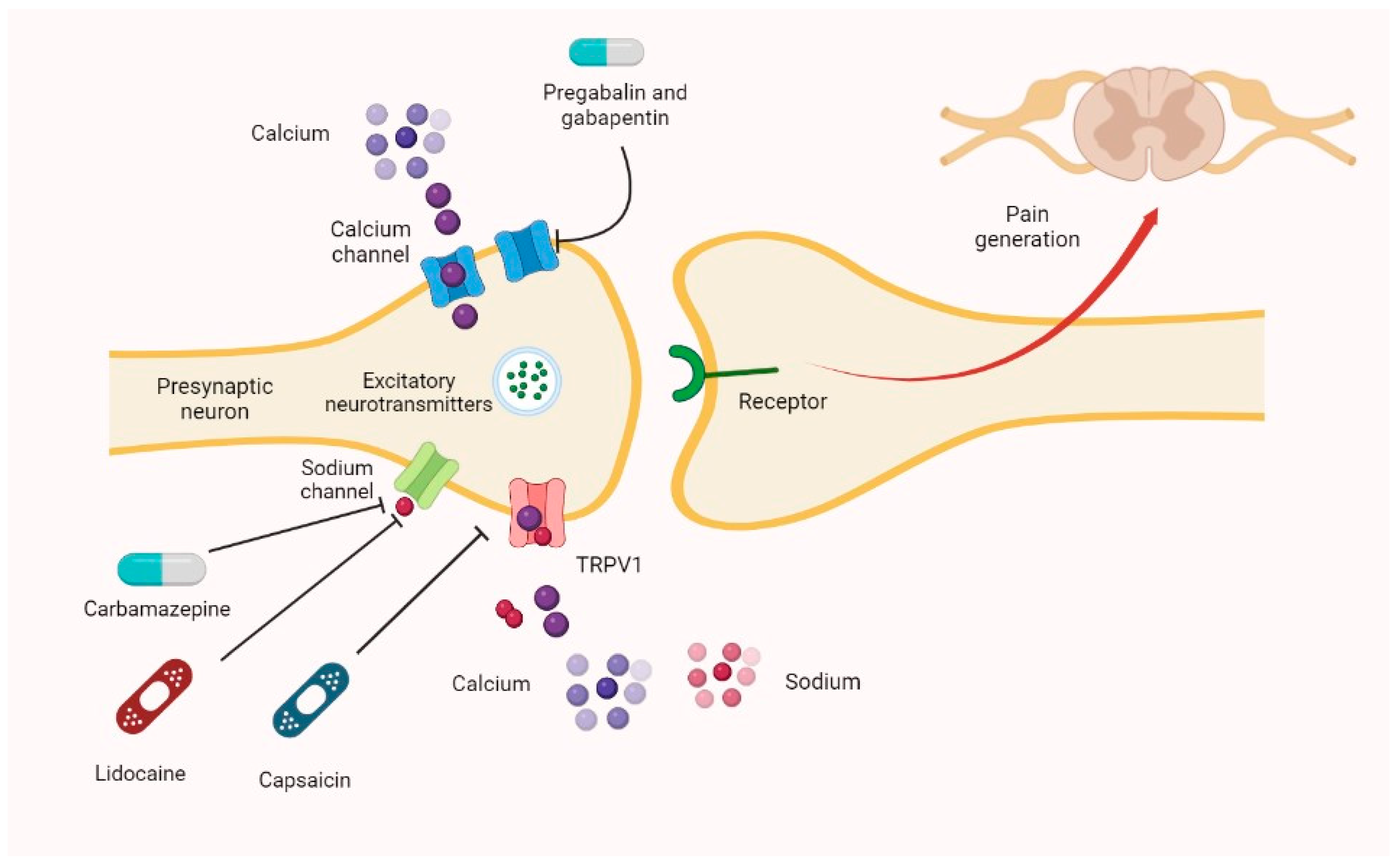
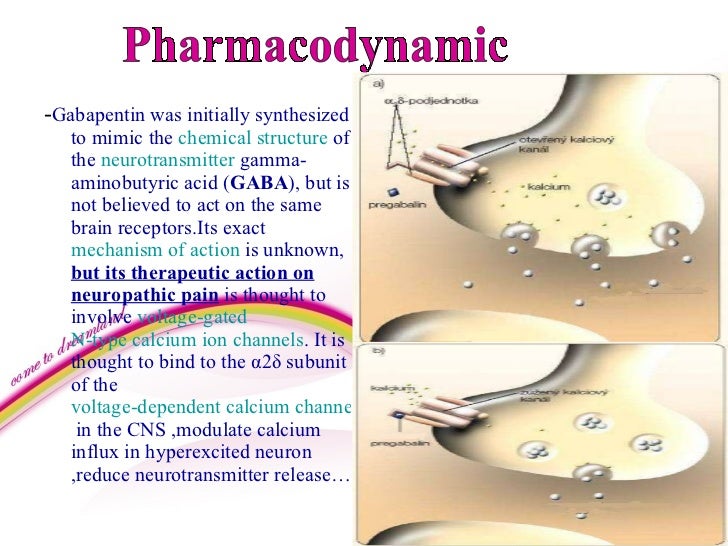
Gabapentin has become popular as a first-line treatment for neuropathic pain because of its efficacy as an antineuropathic agent and relatively benign side-effect profile. However, its Although gabapentinoids are classed as calcium channel blockers, their mechanisms of action are poorly understood. The analgesic effect in neuropathic pain is well evidenced but the role in postoperative pain is less certain. This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. This activity also provides clinicians with the necessary skills and tools to treat various types of muscular, neurological, and psychiatric medical conditions Mechanism of action. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce The analgesic effect in neuropathic pain is well evidenced but the role in postoperative pain is less certain. Medline and EMBASE database searches were conducted to identify studies relating to mechanisms of action and effects in experimental animal models of inflammatory and postoperative pain and human models of experimental pain. Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). The focus of perioperative pain management should be to attempt to minimise the nociceptive input and reduce the risk of transition to central sensitisation. Gabapentinoids are being increasingly used as adjuncts for management of perioperative pain. Although gabapentinoids are classed as calcium channel blockers, their mechanisms of action are poorly understood. The analgesic effect in In randomized open clinical trial, the combination of gabapentin with opioid analgesics was shown to provide better relief in neuropathic pain in cancer patients as compared to opioid analgesics alone in terms of reduction in pain intensity for burning and shooting pain at different days of the study. The analgesic effect in neuropathic pain is well evidenced but the role in postoperative pain is less certain. Medline and EMBASE database searches were conducted to identify studies relating to mechanisms of action and effects in experimental animal models of inflammatory and postoperative pain and human models of experimental pain. Mechanism of Action. Although the exact mechanism of action with the GABA receptors is unknown, researchers know that gabapentin freely passes the blood-brain barrier and acts on neurotransmitters. Gabapentin has a cyclohexyl group to the structure of the neurotransmitter GABA as a chemical structure. Mechanism of action: By inhibiting the voltage-gated calcium channels in the CNS, gabapentin reduces the release of excitatory neurotransmitters (mostly noradrenaline, dopamine and serotonin), and therefore decreases epileptogenesis. Clinical effects Mechanism of action. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. In a meta-analysis of trials evaluating the treatment of neuropathic pain, including painful polyneuropathy and spinal cord injury pain, gabapentin was shown to be safe and effective IASP [Finnerup 2015]. Data from meta-analyses support the use of IR gabapentin for reducing pain by more than 50% in diabetic neuropathy Moore 2014, Rudroju 2013. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Several mechanisms of gabapentin have been proposed after neuropathy including an inhibition of NMDA receptors, inhibition of sodium currents and reducing β4a subunit mediated VGCC trafficking (Hara and Sata 2007; Mich and Horne 2008; Yang et al. 2009). Mechanism of Action. Gabapentin's exact mechanism of action is not fully understood, but it is believed to work by reducing abnormal electrical activity in the brain. It is thought to bind to calcium channels, modulating their activity and reducing the release of neurotransmitters involved in seizures and nerve pain. Mechanisms of action. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. Understanding how gabapentin works for pain is crucial for those exploring treatment options for conditions like neuropathic pain, fibromyalgia, and even post-surgical discomfort. The Mechanism of Action. Gabapentin's primary mechanism revolves around its interaction with calcium channels in the nervous system. Gabapentin is an anti-epileptic drug but its use has expanded to treat multiple other diseases including post-herpetic neuralgia, neuropathic pain, and spasticity. The mechanism of action is not fully understood but may be related to gabapentin’s action on calcium channels leading to diminution of excitatory neurotransmitters.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |