Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
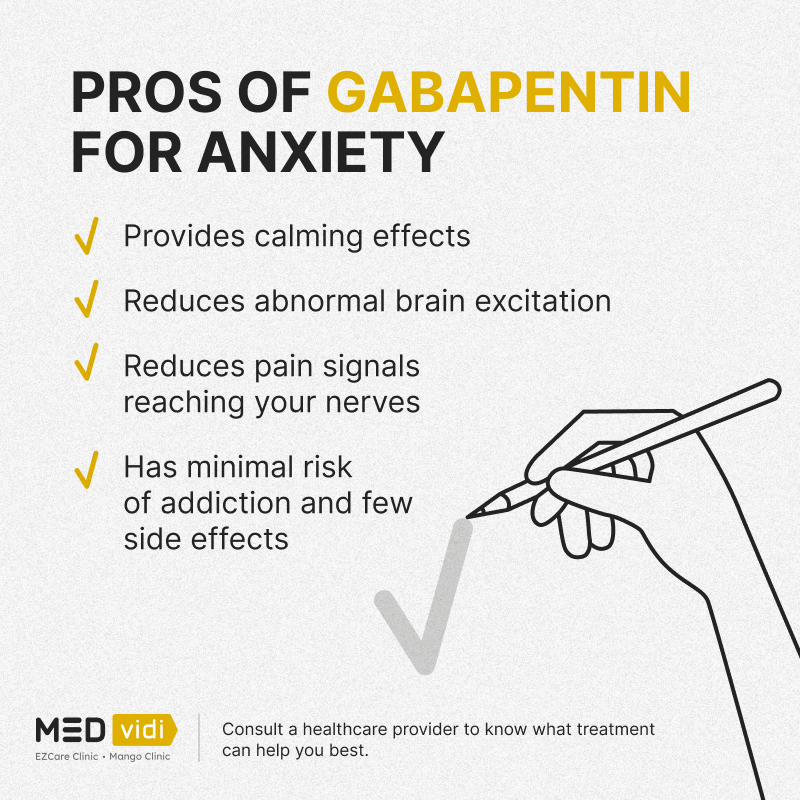
Gabapentin, administered as add-on therapy, was safe and effective in this 12-week, multicenter, placebo-controlled, parallel-group study in 306 patients with refractory partial epilepsy. For patients in each gabapentin treatment group (600, 1,200, 22. Podell M, Fenner WR. Bromide therapy in refractory canine idiopathic epilepsy. J Vet Intern Med 1993;7(5):318-327. 23. Govendir M, Perkins M, Malik R. Improving seizure control in dogs with refractory epilepsy using gabapentin as an adjunctive agent. Aust Vet J 2005;83(10):602-608. 24. Muñana KR. Management of refractory epilepsy. control seizure activity. Gabapentin, a commonly prescribed AED, has shown efficacy in the treatment of epilepsy. This abstract aims to summarize the effectiveness of gabapentin in managing epilepsy based on available clinical evidence. Numerous studies have investigated the efficacy of gabapentin in epilepsy treatment. In people with drug‐resistant focal epilepsy, gabapentin has efficacy as an add‐on treatment. Moderate‐certainty evidence for the outcomes from this review suggests that a dose of 1800 mg/day will reduce seizure frequency by at least 50% in 25.3% of people (95% confidence interval 19.3% to 32.3%). Addition of gabapentin to phenobarbitone and/or potassium bromide increased the interictal period and shortened the post-seizure recovery in some canine epileptics. In some dogs, seizures were prevented completely, while in others there was an increase in interictal period. The short-half life of ga This short-term inpatient study in adults demonstrates that gabapentin monotherapy is an effective and safe treatment for refractory complex partial and secondarily generalized seizures. Eleven dogs diagnosed with refractory idiopathic epilepsy were treated orally with gabapentin for a minimum of three months at an initial dose of 10 mg/kg every eight hours. They were all experiencing episodes of generalised tonic-clonic seizures and had been treated chronically with a combination o Gabapentin (GBP) is one of several novel anti-epileptic drugs effective as an add-on therapy for intractable seizures but has not been studied in patients with cerebral tumours. The efficacy of gabapentin in the management of refractory canine epilepsy has been evaluated in two studies (Platt et al, 2006; Govendir et al, 2005). The first study reported 11 dogs that were already receiving phenobarbital and potassium bromide at therapeutic serum concentrations and were deemed to have poor seizure control (Platt et al, 2006). Parke-Davis' Neurontin was cleared by FDA's Peripheral and Central Nervous System Drugs Advisory Committee at its Dec. 15 meeting. The committee voted unanimously to recommend approval of Neurontin (gabapentin) "as an add-on [therapy] for intractable partial seizures in adults." Gabapentin. Gabapentin is a recent addition to the human anti-convulsant market, which has primarily been used as an adjunctive drug for humans with uncontrolled partial seizures with and without secondary generalization. Gabapentin is well absorbed from the duodenum in dogs with maximum blood levels reached in 1 hour after oral administration. The results of three large, placebo-controlled, multicenter clinical trials of gabapentin as add-on therapy in patients with refractory partial seizures are summarized in a report from the Departments of Neurology and Psychiatry and the International Center for Epilepsy, University of Miami School of Medicine, and the Veterans Affairs Medical The National Institute for Health and Care Excellence (NICE) recommends gabapentin only as adjunctive treatment for refractory focal seizures in children, young people, and adults and for treatment of children and young people with benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, or late‐onset childhood occipital epilepsy When compared to placebo, eslicarbazepine, ezogabine, gabapentin, lacosamide, levetiracetam, perampanel, pregabalin, tiagabine, topiramate, and zonisamide, vigabatrin had one of the highest odds of 50% reduction in seizures and one of the highest odds of seizure freedom in patients with refractory partial-onset seizures. Gabapentin may be an effective adjunctive medication in children with refractory partial seizures. Behavioral side effects were reversible when the drug was discontinued and were most prominent in the mentally retarded. This therapy may improve if combination therapy with potassium bromide (KBr) is used. More recently, several human drugs, such as gabapentin and levetiracetam, have been evaluated for seizure therapy in veterinary patients. This article will discuss anticonvulsant drug use in dogs with so-called 'refractory' or 'intractable' epilepsy. These results demonstrate that gabapentin has anticonvulsant activity and is well tolerated when administered as monotherapy in patients with refractory partial seizures. Get full access to this article There are two clinical reports of gabapentin use as an add-on drug for dogs with refractory epilepsy. Overall, the responder rate of these dogs was between 41% and 55%. Because of its short t ½, gabapentin probably needs to be administered at least every 8 hrs, and possibly every 6 hrs, in order to maintain serum gabapentin concentrations The second study evaluated 17 dogs with refractory seizures that were administered gabapentin at a dose of 35 to 50 mg/kg/day divided twice or three times daily, also in conjunction with phenobarbital and potassium bromide (16 dogs) or phenobarbital alone (1 dog).9 This study found no significant decrease in the number of seizures over the Gabapentin (GBP, Neurontin), a new antiepileptic drug (AED) with a novel mechanism of action, exhibits low acute toxicity in mice, rats, and monkeys, and is not teratogenic. GBP pharmacokinetics are simple and predictable; GBP is eliminated by urinary excretion, is not protein-bound or metabolized,
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |