Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
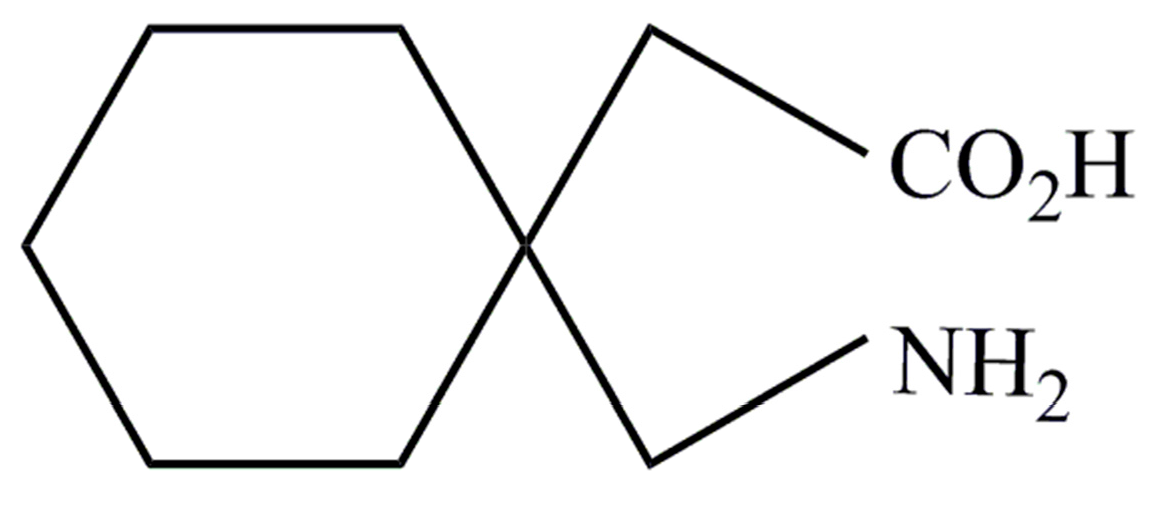
Gabapentin controlled release tablets were evaluated as per the specified limit of USP to meet the quality of formulation. 2 Tablets were evaluated for individual and average weight variations, thickness, hardness, assay and in vitro drug release. Individual and average weight variations were measured in the Single Pan electronic balance Gabapentin is an anti-epileptic drug indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults with epilepsy. Gabapentin exists in The present research work was an attempt to Formulate and Evaluate Gabapentin Gastroretentive mucoadhesive tablets to prolong gastric residence time and increase drug absorption further increasing the bioavailability. The tablets were prepared by direct compression method using mucoadhesive polymers like Gabapentin comes in several dosage forms—tablets, capsules, and oral solutions—each with its unique formulation of active and inactive ingredients. Understanding these forms can help in choosing the right option based on individual needs. Gabapentin gastroretentive tablets: Food increases absorption of the drug formulation; tablets swell upon contact with gastric fluid to a size that promotes gastric retention for approximately 8–10 hours when taken with a meal. The tablet form can be prepared by spray-coating gabapentin with a binder solution and compressing the spray-coated gabapentin into non-friable, stable tablets. This method is particularly The results suggested that direct compression is a suitable method to formulate controlled release Gabapentin tablets and it can perform therapeutically better than conventional immediate release dosage form. TEVA-GABAPENTIN capsules are supplied as follows: 100 mg capsules: White opaque cap and body hard gelatin capsules, size #3, with N and "100" printed in blue ink on opposing cap and body portions of the capsules. 300 mg capsules: Yellow opaque cap and body hard gelatin capsules, size #1, with N and The objective of the present study was to develop a pharmaceutically equivalent, stable, robust, cost effective and quality improved formulation of Gabapentin controlled release tablets by US-A-6054482 discloses that the preparation and long-term storage of gabapentin and its pharmaceutically acceptable salts present problems since (i) during the preparation the compounds show considerable variations without apparent reason; (ii) very pure gabapentin, when stored long term, shows differing stabilities; and (iii) a toxic lactam (II) is formed when the gabapentin degrades. Administer NEURONTIN orally with or without food. NEURONTIN capsules should be swallowed whole with water. Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic acid with a molecular formula of C9H17NO2 and a molecular weight of 171.24. The structural formula of gabapentin is: Gabapentin is Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. prescriptions dispensed for gabapentin nited States were in the U approximately 29.6 million in 2010, 56.9 million in 2015, 69.0 million in 2020, and 73.1million in 202 4. Gabapentin is available in various dosage forms and strengths, including 100, 300 and 400 milligram, capsules; 600 and 800 milligram tablets; and 250 milligrams/5 mL oral Capsules and tablets: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Use scored 600 or 800 mg tablets that are broken in half within 28 days of breaking the tablet. Oral solution: Store refrigerated at 2°C to 8°C (36°F to 46°F). Gabapentin Images. gabapentin 800 mg; gabapentin 300 mg; gabapentin 400 mg This study deals with insilico docking analysis of gabapentin, phosphatidylcholine, and their conjugate to target Phospholipase A2 enzyme followed by formulation and evaluation of phosphatidylcholine conjugated gabapentin loaded nanostructured lipid carriers. YMER || ISSN : 0044-0477 VOLUME 22 : ISSUE 05 (May) - 2023 Gabapentin dosage forms of the invention preferably comprise the gabapentin salt in a daily dosage amount of about 900 to 1800 mg and given in divided doses (three times a day) using 300, 400, 600 or 800 mg tablets/capsules. The starting dose is usually about 300 mg three times a day, but will vary depending on the indication and specific patient. A pharmaceutical formulation form with improved physical and chemical characteristics, comprising gabapentin in tablet form for oral administration. The tablet form can be prepared by spray-coating gabapentin with a binder solution and compressing the spray-coated gabapentin into non-friable, stable tablets. Immediate release tablets of Gabapentin were successfully formulated by employing direct compression method. Preformulation studies of drug were performed; the infrared spectral analysis revealed that there is Solid oral dosage forms of gabapentin are immediate release capsules and immediate-release tablets . The liquid form available in the USA is a simple aqueous solution containing glycerin, xylitol, purified water and artificial flavor . This solution requires refrigeration . Considering its biopharmaceutical properties, a compounded liquid
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |